Improving cyclic stability of polyaniline by thermal crosslinking as electrode material for supercapacitors
Xue Wang,
Dong Liu,
Jinxing Deng,
Xiaojuan Duan,
Jinshan Guo and
Peng Liu*
State Key Laboratory of Applied Organic Chemistry and Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China. E-mail: pliu@lzu.edu.cn; Fax: +86 0931 8912582; Tel: +86 0931 8912582
First published on 9th September 2015
Abstract
The poor cyclic stability of polyaniline restricts its application as an electrode material for supercapacitors, due to the volume changes during the long charge/discharge process. In this work, a thermal crosslinking strategy was developed to improve the cyclic stability of polyaniline electrode materials by thermal treatment of the conventional linear polyaniline (PANI). The heat-treatment conditions including the temperature, atmosphere and time were investigated. Morphology analysis indicated that crosslinked polyaniline (CPANI) had a rougher surface than that of the linear PANI, which could be expected to result in a higher specific area. Compared to the linear PANI, the electrical conductivity of the CPANI increased with the increase of treatment temperature at first and then decreased. The CPANI sample by thermal treatment at 140 °C in air showed the highest electrical conductivity of 6.78 S cm−1. As an electrode material for supercapacitors, the CPANI exhibited an improved electrochemical performance than the linear PANI. After 1300 CV cycles, the CPANI electrode still retained 88.81% of its initial capacitance due to its crosslinking structure.
1. Introduction
Polyaniline (PANI), as one of the most characteristic conducting polymers, has been extensively studied because of its low cost, good environmental stability, doping-dedoping chemistry, high electrical conductivity, and typical redox behaviour.1–3 In the past few years, PANI has been widely utilized for corrosion protection,4,5 sensors,6,7 secondary batteries,8,9 supercapacitors,10,11 and so on. However, its cyclic stability as an electrode material for supercapacitors is unsatisfactory due to the volume change during the long charge/discharge process.12,13The crosslinking reaction, which can provide large conjugated networks and also increase the mechanical stability, is common-used method to improve performance of the polymer. In order to expand the applied range of PANI, a series of crosslinked polyaniline had been prepared via thermal treatment. The structure and conductivities,14–17 mechanical properties,18 thermal properties19–21 and radical scavenging activity22 of the thermally treated polyaniline have been thoroughly investigated. For example, Bhadra and Khastgir estimated the extrinsic and intrinsic structural changes of the crosslinked PANI, which was prepared by heat-treating the electrochemically synthesized polyaniline.23 Pereira da Silva reported about the resonance Raman spectroscopy of the thermally treated polyaniline. The appearance of new peaks related to cyclized “phenazine-like” structures proved the presence of the cross-linking structure.24 The structure of the annealed PANI had been confirmed using solid state NMR.25 The NMR analysis directly indicated the generation of a tertiary amine nitrogen and verified a crosslinking mechanism.
Comparatively speaking, the investigation on the applications of the crosslinked polyaniline is just sprouting recently. Ayad and Zaghlol reported that the thermally crosslinked polyaniline possessed a higher adsorption capacity than that of the conventional PANI for methylene blue (MB).26 Xu's group developed a crosslinked polyaniline using a 1,3,5-tris (phenylamino)benzene (1,3,5-TPAB) unit as the core by chemical polymerization of aniline monomer. The crosslinked polyaniline showed an enhanced electrocatalytic oxidation of ascorbic acid than that of the linear polyaniline.27 But above all, as electrodes materials for supercapacitor, the crosslinked structure is expected to effectively improve the electrochemical performance of polyaniline electrode.
In our previous work,10 the crosslinked PANI were prepared via the chemical oxidative copolymerization in the presence of p-phenylenediamine (PPDA) and triphenylamine (TPA) according to the literature.28 For the crosslinked PANI electrodes, the specific capacitance increased 59%, and the cyclic stability showed a 71% rise than the linear PANI electrode.
Here, we prepared another type of the crosslinked polyaniline (CPANI) as electrode materials for supercapacitor with enhanced electrochemical energy storage performance, by the heat-treating of the conventional linear polyaniline through crosslinking reaction between tertiary amine groups (Scheme 1).26,29,30 The effect of the heat-treating condition (temperature, atmosphere and time) on the morphology and structure, specific surface area, thermal stability, crystal structure and electrical conductivity of the CPANI was investigated, with emphasis on the electrochemical energy storage performance (specific capacitance and electrochemical stability) as electrode materials for supercapacitor.
2. Experimental section
2.1. Materials and reagents
Aniline monomer (Tianjin Guangfu Technology Development Co. Ltd, Tianjin, China) was distilled under reduced pressure. Ammonium persulfate (APS, Tianjin Chemical Reagent Co., Tianjin, China), sulfuric acid (H2SO4, Beijing Chemical Works, Beijing, China) and other reagents were analytical grade and used as received. Double distilled water was used throughout.Carbon black was obtained from Yiping Chemical Factory, Shanghai, China. Polyvinylidenefluoride (PVDF) was received Funuolin New Chemical Materials Co., Ltd Zhejiang, China.
2.2. Preparation of conventional linear polyaniline
The conventional linear polyaniline (PANI) was prepared as follows, with H2SO4 as dopant to avoid the dedoping during the heat-treating. Firstly, aniline monomer (3.64 mL, 0.2 mol L−1) was put into a round bottomed flask (500 mL) containing 100 mL of 0.5 mol L−1 H2SO4 solution and stirred for 30 min. Then, APS (11.41 g, 0.25 mol L−1) pre-dissolved in 100 mL of 0.5 mol L−1 H2SO4 as an oxidant was added dropwise to the above mixture, and the chemical oxidative polymerization was initiated. The reactant mixture was stirred for 10 h in ice-water bath. The precipitate was filtered and washed repeatedly with distilled water and ethanol. The product was dried under vacuum at 50 °C for 24 h.2.3. Thermal crosslinking
In order to obtain the crosslinked polyaniline (CPANI), the above product was heated for 1.0 h at 140, 160, 180 or 200 °C in muffle furnace equipped with a thermostat in air atmosphere.To investigate the effect of the heat-treating conditions on the structure and properties of the CPANI, the PANI was also heated for 0.5 or 1.5 h at 160 °C in air, and for 1.0 h at 160 °C in the inert atmosphere (Ar) (Table 1).
| Samples | PANI | PANI-140 | PANI-160 | PANI-180 | PANI-200 | PANI-160-0.5 | PANI-160-1.5 | PANI-160-Ar |
|---|---|---|---|---|---|---|---|---|
| T (°C) | — | 140 | 160 | 180 | 200 | 160 | 160 | 160 |
| Atmosphere | — | Air | Air | Air | Air | Air | Air | Ar |
| t (h) | — | 1.0 | 1.0 | 1.0 | 1.0 | 0.5 | 1.5 | 1.0 |
| S (m2 g−1) | 14.4 | 16.1 | 15.9 | 16.1 | 20.8 | 16.0 | 18.8 | 18.1 |
| Q/B intensity | 0.953 | 0.877 | 0.851 | 0.711 | 0.809 | 0.696 | 0.772 | 0.793 |
| σ (S cm−1) | 2.21 | 6.78 | 2.53 | 1.58 | 0.16 | 5.80 | 0.67 | 0.23 |
| C (F g−1) | 219.4 | 234.0 | 273.0 | 201.9 | 150.1 | 269.3 | 323.5 | 254.9 |
2.4. Characterizations and measurements
The morphologies of the PANI and CPANI samples were studied by a JEM-1230 transmission electron microscope (TEM, JEOL, Tokyo, Japan) and a S-4800 scanning electron microscope (SEM, Hitachi, Japan).Fourier transform infrared (FT-IR) spectra (Impact 400, Nicolet, Waltham, MA) were obtained using a KBr pellet method over the wavenumber range of 400–4000 cm−1.
Thermogravimetric analysis (TGA) of the samples was recorded using TA Instrument system (Diamond TG/DTA, PerkinElmer Instruments) at a heating rate of 10 °C min−1 in nitrogen atmosphere.
To evaluate the crystal structures of the samples, X-ray diffraction (XRD) patterns were characterized using an X-ray powder diffractometer (XRD, Panalytical X' Pert PRO X-ray Diffractometer) with Cu Kα radiation (40 kV, 30 mA, λ = 0.15418 nm).
The specific surface area (S) of the PANI and CPANI was tested by nitrogen adsorption experiments (ChemiSorb 2750, Micromeritics Instrument Corp.), and calculated by employing the Brunauer–Emmett–Teller (BET) plot.
The electrical conductivities of the PANI and CPANI pellets were recorded utilizing a RTS-2 four-point probe conductivity tester at ambient temperature. The sample pellets were obtained by pressing the powder samples using a pressure of 15 MPa.
The electrochemical performance of the PANI and CPANI electrodes were recorded on a conventional three-electrode system with a working electrode (stainless steel mesh), a platinum counter electrode, and a standard calomel reference electrode (SCE). The working electrode was obtained by that the homogeneous mixture containing active materials (PANI or CPANI powders), carbon black, and polyvinylidenefluoride (PVDF) with a mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in DMF were loaded on a stainless steel mesh. Galvanostatic charge–discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests of the PANI and CPANI samples electrodes were performed on a CHI660E electrochemical workstation (CH Instruments) in the potential range of −0.2 to +0.8 V using a 1.0 M H2SO4 solution as the electrolyte. EIS tests were carried out using an open circuit potential of 0.4 V in the frequency range from 100 kHz to 0.01 Hz with an AC perturbation of 5 mV.
5 in DMF were loaded on a stainless steel mesh. Galvanostatic charge–discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests of the PANI and CPANI samples electrodes were performed on a CHI660E electrochemical workstation (CH Instruments) in the potential range of −0.2 to +0.8 V using a 1.0 M H2SO4 solution as the electrolyte. EIS tests were carried out using an open circuit potential of 0.4 V in the frequency range from 100 kHz to 0.01 Hz with an AC perturbation of 5 mV.
3. Results and discussion
3.1. Morphology and specific surface area analysis
The morphology of the CPANI samples, prepared by heat treatment of the linear polyaniline at different temperatures, times and atmospheres, was compared with the linear polyaniline by TEM and SEM techniques, as showed in Fig. 1 and 2. Fig. 1a and 2a show a typical irregular morphology of the linear PANI. After heat treatment, the CPANI samples displayed a rougher surface with hairy surface. The sample PANI-160-Ar prepared in Ar showed more clear hairy-surface than that of the sample PANI-160 prepared in air at the same temperature with the same time (Fig. 2c and h).The specific surface areas of all samples were summarized in Table 1. It increased with a maximum value of 20.8 m2 g−1 for the PANI-200, with increasing the heating temperature or time. Compared to the PANI-160, the PANI-160-Ar by heating in Ar stream showed a rougher surface with higher specific surface areas. The high specific surface area can increase the reaction sites, which is advantageous to improve the electrical and electrochemical properties.
The specific surface area data are different from the phenomena reported previously, in which the thermal treatment had no effect on the morphology of PANI sample.22 This might be determined by the morphology and size of the conventional PANI materials used. For the PANI nanoparticles, the thermal treatment led to the aggregation.26 The dopant used might be another influencing factor. The dopant HCl could be dedoped during the heat-treating, and the dedoped PANI seems easier to be crosslinked via heat-treating. Here, H2SO4 was used as dopant to avoid the dedoping during heat-treating, thus the higher electrical conductivity should be achieved.
3.2. Structure analysis
The FT-IR spectra of the PANI and CPANI samples are illustrated in Fig. 3. All samples showed a prominent peak at 1150 cm−1 derived from S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the dopant sulfuric acid. The characteristic peak at 1573 cm−1 is assigned to the C
O of the dopant sulfuric acid. The characteristic peak at 1573 cm−1 is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the quinonoid rings. Both samples showed a clear shoulder peak at 1573 cm−1 on the higher wavenumber side related to the NH2+ deformations.22 The absorbance peak at 1477 cm−1 is assigned to the C
C stretching of the quinonoid rings. Both samples showed a clear shoulder peak at 1573 cm−1 on the higher wavenumber side related to the NH2+ deformations.22 The absorbance peak at 1477 cm−1 is assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzenoid ring. The peaks at 1380 and 1307 cm−1 are corresponded to the C–N stretching of a quinonoid ring neighbourhood and of a secondary aromatic amine,16 respectively.
C stretching of benzenoid ring. The peaks at 1380 and 1307 cm−1 are corresponded to the C–N stretching of a quinonoid ring neighbourhood and of a secondary aromatic amine,16 respectively.
 | ||
| Fig. 3 FT-IR spectra of the PANI and CPANI samples treated under different temperature (a), and the CPANI samples treated for different time or under Ar atmosphere (b). | ||
The intensity ratios (Q/B) of the peaks at 1573 and 1477 cm−1 (ratio of quinonoid to benzenoid ring) has been used to estimate the chemical structure change in the treated PANI.22,23,25,26 The Q/B value of the thermally treated PANI decreased firstly and then increased with increasing the treating temperature with a minimum Q/B ratio for the PANI-180 sample (Table 1), indicating a maximum conversion of quinonoid ring to benzenoid ring attributed to the crosslinking reaction (Scheme 1).17 The increased Q/B ratio of the PANI-200 sample implied the oxidation of PANI from benzenoid ring to quinonoid ring.23,29 Furthermore, the PANI-160-Ar sample showed a lower Q/B ratio than that of the PANI-160, meaning a higher crosslinking degree, which is in agreement with morphology and specific surface area data. This is due to that the presence of oxygen in air could only accelerate the crosslinking process.17
Fig. 4 shows the XRD patterns of the PANI and CPANI samples. The diffraction peaks at 2θ = 15.6°, 21.1° and 25.6° are assigned to the (011), (020) and (200) crystal planes of the emeraldine salt (ES) form of polyaniline, respectively.30 The XRD patterns of both the PANI and the CPANI samples showed a similar curve, implying the amorphous structure. However, the diffraction peak intensity of the CPANI samples is lower than the PANI, due to that the 3-D crosslinking structure between the chains of polyaniline decreases the crystallinity, and the amorphous state of the samples becomes evident.21 The XRD patterns can assert that a crosslinking reaction happened during the thermal treatment.
 | ||
| Fig. 4 XRD patterns of the PANI and CPANI samples treated under different temperature (a), and the CPANI samples treated for different time or under Ar atmosphere (b). | ||
3.3. Thermal stability
The TGA curves of the PANI and CPANI samples are presented in Fig. 5. It can be observed that all samples exhibited the six continuous steps of weight loss. Among them, the three severe losses occurred at <120 °C, 200–350 °C, and 450–600 °C respectively, were summarized in Table 2. The initial weight loss at <120 °C can be attributed to the loss of moistures. | ||
| Fig. 5 TGA curves of the PANI and CPANI samples treated under different temperature (a), and the CPANI samples treated for different time or in Ar atmosphere (b). | ||
| Weight losses (%) at different temperatures | PANI | PANI-140 | PANI-160 | PANI-180 | PANI-200 | PANI-160-0.5 | PANI-160-1.5 | PANI-160-Ar |
|---|---|---|---|---|---|---|---|---|
| <120 °C | 9.42 | 7.45 | 7.14 | 7.08 | 5.95 | 7.12 | 6.36 | 6.14 |
| 200–350 °C | 20.69 | 23.23 | 21.61 | 21.26 | 20.48 | 21.02 | 21.00 | 20.69 |
| 450–600 °C | 13.87 | 13.41 | 13.81 | 10.03 | 9.19 | 13.50 | 10.41 | 11.69 |
The weight loss in 200–350 °C may be due to the removal of H2SO4 dopant. This is the main reason for the lowest electrical conductivity of the PANI-200 sample. With the heating temperature near to 200 °C or heating for longer time, the dedoping of H2SO4 dopant is more significant, therefore the lower electrical conductivity was resulted. Furthermore, the protonation with the bivalent counter-ions may undergo a more complex transformation at elevated temperature.16 This would also explain the better stability and improved resistivity against deprotonation, compared with hydrochloric acid dopant.
The weight loss in 450–600 °C is ascribed to the thermal decomposition of PANI. In addition, the relatively slight weight losses within 120–200 °C, 350–450 °C, and 600–800 °C may be attributed to the inter-chain crosslinking, the removal of bound H2SO4 dopant and the carbonization of samples, respectively.23 It is clear that the thermally treated PANI showed a lower weight loss than the linear PANI, indicating the improved thermal stability of the crosslinked polyaniline.
3.4. Electrical conductivity
The electrical conductivity can indicate the change in the polyaniline structure. The electrical conductivity of the PANI and CPANI samples are summarized in Table 1. The electrical conductivity of the linear PANI was 2.21 S cm−1. After the thermal treatment at 140 °C, the electrical conductivity of the sample increased to 6.78 S cm−1 due to the reduction of moisture28 in the PANI-140 sample and the formation of the large crosslinked conjugated structure. In addition, a suitable temperature for heat-treating can increase the molecular chain mobility and improve the molecular chain ordering, thereby enhance the electrical conductivity of the sample. Then the electrical conductivity of the CPANI samples exhibited a heavy loss caused by low conjugation length with increasing temperature and time of thermal treatment17 and low crystallinity23 produced by the crosslinking reaction. Moreover, the PANI-160-Ar sample by heat-treating in Ar atmosphere had a lower electrical conductivity than that treated in air (PANI-160). This could be due to that more inter-chain crosslinking structure formed in Ar atmosphere greatly decreased the conjugation length and the crystallinity of the samples.3.5. Electrochemical performance
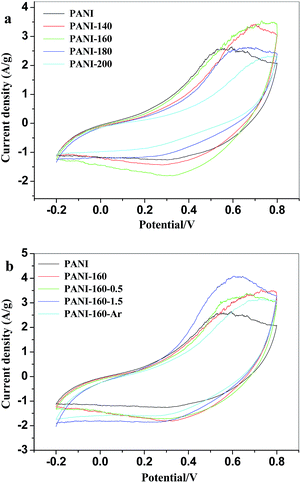
To investigate the electrochemical performance of the CPANI, the CPANI electrodes were tested using GCD, CV and EIS techniques. The specific capacitance (Cm) of the PANI and CPANI electrodes was calculated using equation:| Cm = (∫IdV)/(mVν) |
These tests demonstrated that the crosslinking via the heat-treating could improve efficiently the electrochemical performance of the PANI electrode materials for supercapacitor, especially for the electrochemical cyclic stability. It could be used to resolve the fatal problem which restricts the practical application of the conductive polymers as the electrode materials for supercapacitor, as reported previously.10 Besides, the crosslinking strategy via the heat-treating developed in the present work possesses unique advantage, in comparison with the chemical crosslinking copolymerization method. The facile crosslinking via the heat-treating is expected to be used for the crosslinking of polyaniline materials in any form, such as bulk, film or composite. So the crosslinking via the heat-treating proposed here is more practical.
4. Conclusions
In summary, a more practical crosslinking technique was proposed to synthesize novel crosslinked polyaniline (CPANI) as the electrode materials for supercapacitor, via the facile thermal treatment of the conventional linear polyaniline (PANI). The CPANI samples exhibited a rougher surface than that of the untreated PANI, and enhanced electrical conductivity due to the high chain-ordering and the reduction of moisture. Supercapacitor based on the CPANI electrodes possessed an enhanced electrochemical energy storage property, especially for the electrochemical cyclic stability. In contrast to the untreated PANI electrode, the CPANI electrode remained as high as 88.81% of its initial capacitance after 1300 CV cycles at scan rate of 100 mV s−1, attributed to the effect of the dense crosslinked structure formed by the thermal treatment of the linear polyaniline. So the crosslinking via the heat-treating proposed here is more practical to improve efficiently the electrochemical performance of the PANI electrode materials for supercapacitor.Acknowledgements
This work was supported by the Natural Science Foundation of Gansu Province (Grant No. 1107RJZA213) and the Science and Technology Project of Chengguan District, Lanzhou City (Grant No. 2014-2-1).Notes and references
- W. S. Huang, B. D. Humphrey and A. G. MacDiarmid, J. Chem. Soc., Faraday Trans., 1986, 82, 2385 RSC.
- A. G. MacDiarmid, J. C. Chiang, A. F. Richter and A. J. Epstein, Synth. Met., 1987, 18, 285 CrossRef CAS.
- E. T. Kang, K. G. Neoh and K. L. Tan, Prog. Polym. Sci., 1998, 23, 277 CrossRef CAS.
- F. Chen and P. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 2694 CAS.
- J. M. Yeh, S. J. Liou, C. Y. Lai and P. C. Wu, Chem. Mater., 2001, 13, 1131 CrossRef CAS.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, J. Am. Chem. Soc., 2003, 125, 314 CrossRef CAS PubMed.
- S. Virji, J. Huang, R. B. Kaner and B. H. Weiller, Nano Lett., 2004, 4, 491 CrossRef CAS.
- J. M. Jeong, B. G. Choi, S. C. Lee, K. G. Lee, S. J. Chang, Y. K. Han, Y. B. Lee, H. U. Lee, S. Kwon, G. Lee, C. S. Lee and Y. S. Huh, Adv. Mater., 2013, 25, 6250 CrossRef CAS PubMed.
- W. D. Zhou, Y. C. Yu, H. Chen, F. J. DiSalvo and H. D. Abruna, J. Am. Chem. Soc., 2013, 135, 16736 CrossRef CAS PubMed.
- X. Wang, J. X. Deng, X. J. Duan, D. Liu, J. S. Guo and P. Liu, J. Mater. Chem. A, 2014, 2, 12323 CAS.
- J. X. Deng, X. Wang, J. S. Guo and P. Liu, Ind. Eng. Chem. Res., 2014, 53, 13680 CrossRef CAS.
- X. W. Li, H. Zhang, G. C. Wang and Z. H. Jiang, J. Mater. Chem., 2010, 20, 10598 RSC.
- I. Kovalenko, D. G. Bucknall and G. Yushin, Adv. Funct. Mater., 2010, 20, 3979 CrossRef CAS PubMed.
- G. M. Do Nascimento, C. H. B. Silva and M. L. A. Temperini, Polym. Degrad. Stab., 2008, 93, 291 CrossRef CAS PubMed.
- M. Trchova, P. Matejka, J. Brodinova, A. Kalendova, J. Prokes and J. Stejskal, Polym. Degrad. Stab., 2006, 91, 114 CrossRef CAS PubMed.
- M. Trchova, I. Sedenkova, E. Tobolkova and J. Stejskal, Polym. Degrad. Stab., 2004, 86, 179 CrossRef CAS PubMed.
- Y. Kieffel, J. P. Travers, A. Ermolieff and D. Rouchon, J. Appl. Polym. Sci., 2002, 86, 395 CrossRef CAS PubMed.
- O. Oka, O. Kiyohara and S. Morita, Synth. Met., 1993, 55, 999 CrossRef CAS.
- T. Hagiwara, M. Yamaura and K. Iwata, Synth. Met., 1988, 25, 243 CrossRef CAS.
- L. Ding, X. Wang and R. V. Gregory, Synth. Met., 1999, 104, 73 CrossRef CAS.
- C. H. Chen, J. Polym. Res., 2002, 9, 195 CrossRef CAS.
- A. V. Nand, S. Ray, M. Gizdavic-Nikolaidis, J. Travas-Sejdic and P. A. Kilmartin, Polym. Degrad. Stab., 2011, 96, 2159 CrossRef CAS PubMed.
- S. Bhadra and D. Khastgir, Polym. Degrad. Stab., 2008, 93, 1094 CrossRef CAS PubMed.
- J. E. Pereira da Silva, D. L. A. de Faria, S. I. Córdoba de Torresi and M. L. A. Temperini, Macromolecules, 2000, 33, 3077 CrossRef CAS.
- R. Mathew, B. R. Mattes and M. P. Espe, Synth. Met., 2002, 131, 141 CrossRef CAS.
- M. Ayad and S. Zaghlol, Chem. Eng. J., 2012, 204, 79 CrossRef PubMed.
- S. Chen, L. Xu, Y. Yang, B. Li and J. Hou, Anal. Methods, 2011, 3, 2374 RSC.
- Y. Yang, S. Chen and L. Xu, Macromol. Rapid Commun., 2011, 32, 593 CrossRef PubMed.
- V. M. Mzenda, S. A. Goodman, F. D. Auret and L. C. Prinsloo, Synth. Met., 2002, 127, 279 CrossRef CAS.
- H. K. Chaudhari and D. S. Kelkar, Polym. Int., 1997, 42, 380 CrossRef CAS.
- Q. Wu, Y. Xu, Z. Y. Yao, A. R. Liu and G. Q. Shi, ACS Nano, 2010, 4, 1963 CrossRef CAS PubMed.
- J. E. P. da Silva, S. I. C. de Torresi and M. L. A. Temperini, J. Braz. Chem. Soc., 2000, 11, 91 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |