Selective isomerization of epoxides using magnetically recyclable ZSM-5 zeolite catalyst
Phan Huy
Hoang
*a and
Bach
Nguyen Xuan
b
aSchool of Chemical Engineering, Hanoi University of Science & Technology, No.1 Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
bHanoi Pedagogical University 2, Nguyen Van Linh Street, Phuc Yen District, Vinh Phuc Province, Vietnam
First published on 8th September 2015
Abstract
The application of magnetically recyclable ZSM-5 zeolite (MZZ) for epoxide isomerization reaction was presented. This catalyst exhibited a high catalytic efficiency for liquid phase rearrangement of 2-methyl-2,3-epoxybutane and styrene oxide, as well as high efficiency in separation, recycling with long lifetime and no significant loss of catalytic activity. The influences of reaction conditions such as solvent, reaction time, catalyst loading and temperature on the efficiency of epoxide isomerization were also investigated. Moreover, it was noticeable that for the epoxides investigated the high catalytic activity observed over the MZZ catalyst (conversion over 98% after 2 h reaction for both of two epoxides) was accompanied by the achievement of high selectivities, typically 79% and 82% for 2-methyl-2,3-epoxybutane and styrene oxide respectively, towards desired products with commercial applications.
Introduction
Epoxides are very important and versatile intermediates in organic synthesis since they can be readily transformed into a wide range of organic compounds.1,2 Isomerization of epoxides over acid catalysts is an important process to yield carbonyl compounds and their halogenated derivatives,3 which are valuable compounds or intermediates used for production of fragrances, pharmaceuticals, insecticides, fungicides, and herbicides. Several catalytic systems have been developed for the isomerization of epoxides to carbonyl compounds.4–8 Acid-catalyzed epoxide rearrangement reaction usually leads to ketones and aldehydes, whereas the base-catalyzed reaction forms allylic alcohols as main products.9 Using of homogeneous catalysis presents numerous drawbacks such as loss of the catalyst, corrosivity, toxicity and generation of contaminated streams in most cases. Thus many efforts have been conducted to find out heterogeneous catalysts to solve these problems. For this reason heterogeneous catalysts such as alkaline earth metal sulfonates, mixed-metal oxides, silica–alumina gels, natural silicates, Nafion-H and zeolites have been tested for isomerization of epoxide.10–14 Nevertheless, the competitive aldol condensation or polymerization of aldehydes and rapid deactivation of catalysts by aggregation or coke formation are still difficult problems at the industrial scale.3,9,15 The use of conventional catalysts often results in the formation of aldol condensation products and mixtures of ketones and aldehydes as by-products. The numerous molecules formed by aldol condensation are the first step in the formation of coke thus limiting the lifetime of catalysts.The use of zeolitic catalysts for the isomerization of epoxide showed main advantage due to their well defined pore system compared with homogeneous systems or other heterogeneous catalysts such as metal oxides, silica–alumina gels or metal sulfates.16,17 Indeed, zeolite materials in their proton form have been found to catalyze this type of reactions.16,18 Moreover, the well defined pore system of zeolites may suppress the side reactions mentioned above.9,19 Hence, high selectivities to aldehydes could be achieved in the isomerization of styrene oxide and derivatives over various acid zeolites. Besides, the practical application of catalysts in liquid phase reactions is hindered by both high costs and difficulties in catalyst separation and recycling. Many different approaches have been suggested to solve the difficulties in catalyst separation, such as filtration, polymeric supports and other new separation techniques.20–22 Each separation method has its own limitations of cost, efficiency or generation of secondary waste. Magnetic separation has emerged as a robust, highly efficient and rapid catalyst separation tool as it provides a convenient method for removing and recycling magnetized species by applying an appropriate magnetic field.20,21,23 This approach may prevent the agglomeration of the catalyst particles during recovery and can increase the durability of the catalysts.24
We were, therefore, motivated that magnetically recyclable ZSM-5 zeolite that was synthesized by deposition of ZSM-5 zeolite on the surface of silica-coated magnetite particles, could be applied for isomerization of epoxide to overcome the above mentioned problems. These magnetic zeolite particles could take fully the advantages that are offered by both magnetic separation and zeolite catalyst. Herein, we report the application of magnetically recyclable ZSM-5 zeolite for epoxide rearrangement reaction for improve the efficiency of synthesis process in separation, recycling, lifetime and catalytic activity of catalyst.
Materials and methods
Synthesis of core–shell magnetic ZSM-5 zeolite particles
Core/shell-structured magnetic ZSM-5 zeolite (MZZ) was obtained by following reported method that was developed by our own group.20 The uniform magnetite (Fe3O4) particles were synthesized by a coprecipitation method by using FeCl2·4H2O and FeCl3·6H2O as precursors. These Fe3O4 particles were then used as core for fabrication of silica coated magnetite beads (Fe3O4/SiO2). Magnetite particles were added to a solution containing 50 mL of ethanol, 5 mL of distilled water, and 2 g of aqueous ammonia at room temperature and the resulting solution was vigorously stirred for 30 min. Tetraethoxysilane (TEOS, 0.4 mL) was then rapidly added and the solution aged at room temperature for 12 h. Then the Fe3O4/SiO2 nanoparticles were magnetically separated, washed and then dried. The silica coated magnetite particles were then introduced to ZSM-5 synthetic solution for hydrothermal synthesis at 150 °C in 20 h to obtain core–shell magnetic ZSM-5 zeolite (MZZ) catalyst. The synthetic zeolite solution was obtained after aging for 12 h by stirring at room temperature, with a mole ratio of the composition of TEOS/TPAOH/NaAlO2/KOH/H2O = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05
1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85
0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64.500.
64.500.
Epoxide rearrangement reaction
To examine the catalytic efficiency as well as the separation and recycle of the magnetic zeolite particles, the catalytic rearrangements of epoxide were performed. All reactions were carried out under a nitrogen atmosphere in a 100 mL two necked round bottomed flask equipped with a water condenser and a magnetic stirrer. The solvent and the catalyst were initially placed in the flask. In a typical experiment, epoxide (1 g) was directly added to a mixture of magnetic zeolite catalyst and solvent (10 mL) and the mixture was stirred for the appropriate reaction time. The employed catalyst loading was 5% w/w over reactant (1 g of epoxide and 50 mg of catalyst). When the reaction was complete, the catalyst was separated by external magnetic field and washed several times, then the solvent was removed to get the products.4Characterization
X-ray diffraction (XRD) was performed on a Rigaku D/max lllC (3 kW) with a θ/θ goniometer equipped using a Cu-Kα radiation generator. Transmission electron microscopy (TEM) was performed using a JEM 2100F, JEOL, Japan, operating at 200 kV. Scanning electron microscopy (SEM) was performed using a JSM-7000F, JEOL, Japan. Brunauer–Emmett–Teller (BET) analysis was used to determine the surface areas of the samples. The Si/Al ratio of the samples was determined by induced coupled plasma-atomic emission spectroscopy (ICP-AES) with a Varian VISTA-AX equipment. Temperature programmed desorption of ammonia (NH3-TPD) was performed on a conventional apparatus equipped with a thermal conductivity detector to determine the acidic properties of the catalysts. The sample was pretreated at 500 °C for 2 h with a N2 flow and then cooled down to ambient temperature. NH3 was supplied to the system followed by flushing with N2 at 150 °C for 1 h. The TPD profile was obtained by heating the sample from 150 to 500 °C at a rate of 15 °C min−1. The NH3 desorbing between 150 and 500 °C was trapped in boric acid and then titrated with a standard H2SO4 solution.3,15 According to the individual peak area, the amount of NH3 can be calculated and then converted into the acid concentrations with the assumption of one NH3 molecule per acid site.The structures of products obtained from the rearrangement reactions were confirmed by their 1H-NMR spectra. The yield of the aldehyde product was also determined by 1H-NMR with trimethyl-phenyl-silane (PhSiMe3) as internal standard, which was added after the synthetic work-up just prior to the NMR analysis. The 1H NMR spectra were performed in CDCl3 on a Bruker DMX600 spectrometer with a 7788 Hz spectral width, a relaxation delay of 1.0 s, and a pulse width of 30°. The catalyst reaction products were also analyzed with a VARIAN 3800 gas chromatograph equipped with a capillary column (HP-FFAP) with dimensions 60 m × 0.32 mm, using a flame ionization detector (FID). Identification of the different reaction products was performed by mass spectrometry (VARIAN SATURN 2000) using standard compounds.
Results and discussion
Synthesis of core–shell magnetic ZSM-5 zeolite (MZZ) particles
Core/shell-structured magnetically recyclable ZSM-5 zeolite (MZZ) was obtained by following reported method that was developed by our own group.20 The MZZ particles were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and scanning electron microscopy (SEM) and shown in Fig. 1. Fig. 1A showed XRD patterns of the as-synthesized MZZ particles. Two sets of diffraction peaks appeared in their XRD patterns, one set of peaks was consistent with the magnetite Fe3O4 crystals (JCPDS card No. 19-0629), while the other set of peaks matched well with ZSM-5 zeolite (JCPDS card No. 44-0002).20 This result indicated that core–shell magnetic ZSM-5 zeolite (MZZ) particles have been successfully prepared. The TEM images (Fig. 1B and C) showed that the as-obtained magnetic zeolite particles were homogeneous in size and morphology with an average diameter of about 300 nm, and each particle just contains one single Fe3O4 core.The chemical composition and some textural properties of the catalysts are shown in Table 1. The BET surface area and external surface area of magnetic ZSM-5 zeolite were calculated to be 428.2 and 86.8 m2 g−1, respectively. These results suggest that these MZZ particles had high surface area, and was higher than conventional ZSM-5 zeolite. It could be explained that the as-obtained MZZ particles after coating of zeolite layer on Fe3O4/SiO2 core had the rough surfaces (see Fig. 1D and E), presumably due to the random growth of the ZSM-5 zeolite particles.20 These rough surfaces could provide high surface area and large external surface areas for the adsorption of guest species. Moreover, core–shell structure with void space generated from shrinkage of silica coated magnetite core also could be attributed to contribute to the high surface area of MZZ particles.
| Catalyst | Si/Al (mol mol−1) | Pore diameter (Å) | S BET (m2 g−1) | S EXT (m2 g−1) | V pore (cm3 g−1) | Acidity (mmol g−1) |
|---|---|---|---|---|---|---|
| Magnetic ZSM-5 zeolite | 86 | 5.5 | 428.2 | 86.8 | 0.219 | 0.48 |
As also seen in Table 1, MZZ samples possessed Si/Al molar ratios slightly higher than that corresponding to the synthesis gels (Si/Al = 86). The acidity of MZZ sample has been investigated by NH3-TPD measurement. The total amount of ammonia desorbed (mmol g−1) may be related to the number of acid sites. It's seen that the acidity of MZZ was relatively high with acid concentration of 0.48 mmol g−1, and comparable to the results reported in the literature.3,15,26 It is well known that zeolites have been widely used in ion exchange, adsorbents, separation and catalysis for organic synthesis. The magnetic separation has emerged as a robust, highly efficient separation tool with many advantages. It provides a convenient method for removing and recycling by applying an appropriate magnetic field.20,24 Thus in this current work, we applied as-synthesized MZZ catalyst for liquid phase epoxide isomerization reaction to exploit the advantages of these particles for the organic synthesis.
Catalytic and recycle efficiency of core–shell magnetic ZSM-5 zeolite
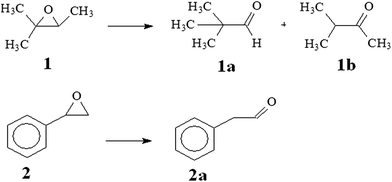
Isomerization of epoxides over acid catalysts is an important process to yield carbonyl compounds and its halogenated derivatives,3 which are valuable compounds or intermediates used for the preparation of perfumes, synthetic food flavourings and pharmaceuticals. However, the competitive aldol condensation or polymerization of aldehydes and rapid deactivation of catalysts by coke formation are still difficult problems at the industrial scale.3 Thus core–shell magnetic ZSM-5 zeolite catalyst was applied for epoxide isomerization reaction in order to assess the advantages offered by both magnetic separation and zeolite catalyst. Two epoxides having different molecular geometry have been selected as model compounds: 2-methyl-2,3-epoxybutane and styrene oxide (Scheme 1).It is known that the solvent plays an important and sometimes decisive role in the catalytic behavior of a catalyst.25 In most of organic reactions, solvents must be added to dilute reactants in order to control the side reactions and prevent the catalyst deactivation. Therefore, first attempt we studied the effect of the solvent on epoxide isomerization by doing several isomerization reactions of 2-methyl-2,3-epoxybutane and styrene oxide with several solvents and under identical conditions. Table 2 showed the conversion results obtained using 0.05 g of MZZ, after various reaction time at 40 °C. From the Table 2, it is evident the importance of the correct selection of solvents. The results indicated that the conversions of 2-methyl-2,3-epoxybutane and styrene oxide in presence of magnetic ZSM-5 zeolite catalyst (MZZ) were different with different solvents used. The conversions were low in methanol even after 4 h, presumably due to the weakening of the acidity of the zeolite catalytic sites by hydrogen bonding to methanol, hindering the participation of the proton in the solvolysis of the oxirane ring.4,25 Moreover, the low selectivity towards aldehyde obtained might be related with the basic character of the methanol which could accelerate the formation of alcohol byproduct.9,25,26 The conversion yield was high after just 1 h in solvents of acetone and dichloromethane. It's observed that the higher solvent polarity the lower conversion of epoxide. This behavior may be explained by the decrease in the substrate concentration within the pores of the catalyst as the polar nature of the solvent increases.25 Indeed, the higher the solvent polarity, the lower the concentration of the substrate in the vicinity of the active sites and the lower the epoxide conversion would be, as indicated by the experimental results. However, a low polar solvent such as toluene showed slow reaction with the lower epoxide conversion than acetone. This catalytic performance over magnetic ZSM-5 zeolite could be more related with intraparticle diffusion problems, arising from the limited pore size of the zeolitic material, than with the polarity effects.25
| Solvent | 2-Methyl-2,3-epoxybutane isomerization | Styrene oxide isomerization | |||
|---|---|---|---|---|---|
| Reaction time, h | Conversionb, % | Selectivityb, % | Conversion, % | Selectivity, % | |
| a Reactant (1 g) and MZZ catalyst (0.05 g) in 10 mL solvent at desired temperature under N2 atmosphere. b Calculated from GC and 1H NMR. c Selectivity of 1b. | |||||
| Methanol | 1 | 34.7 | 40.4 (15.2)c | 31.4 | 65.7 |
| 2 | 50.2 | 40.9 (15.0) | 46.8 | 66.1 | |
| 4 | 60.3 | 40.0 (15.8) | 54.5 | 65.5 | |
| Dichloromethane | 1 | 84.5 | 75.2 (14.0) | 80.1 | 80.0 |
| 2 | 95.8 | 74.8 (14.6) | 91.7 | 79.1 | |
| 4 | 97.1 | 75.7 (14.1) | 96.0 | 79.5 | |
| Acetone | 1 | 86.3 | 77.8 (12.7) | 84.6 | 82.8 |
| 2 | 96.9 | 79.0 (12.0) | 93.5 | 82.1 | |
| 4 | ∼100 | 78.2 (12.1) | 99.1 | 82.7 | |
| Toluene | 1 | 48.7 | 58.1 (23.5) | 40.2 | 88.7 |
| 2 | 60.9 | 57.3 (23.9) | 53.9 | 88.0 | |
| 4 | 72.4 | 58.0 (24.3) | 63.8 | 88.2 | |
The results also showed that the conversion obtained in the rearrangement of 2-methyl-2,3-epoxybutane over MZZ catalyst was slight higher than that obtained using styrene oxide. It is well established that in addition to the different reactivity of the epoxide, the catalytic performance over MZZ catalyst is also related to the overall accessibility of the substrate to the active sites, which is influenced by the possible presence of both steric and intracrystalline diffusion problems. 2-Methyl-2,3-epoxybutante molecule has a lower dimensions than that of styrene oxide as well as much lower than the diameter of the ZSM-5 zeolite micropores.26 Hence, this molecule could enter more easily the zeolite micropores and thus gave higher reactivity than molecule of styrene oxide. As a result of above findings, acetone was used as the solvent for next studies.
As also seen in Table 2, the conversion yields of epoxide using magnetic ZSM-5 zeolite catalyst (MZZ) increased with the increase of reaction time. In particular, the conversion of 2-methyl-2,3-epoxybutane was 86.3% for reaction time of 1 h and nearly complete (∼100%) for reaction time of 4 h in acetone solvent, with the selectivity of 1a product (2,2-dimethylpropanal) was about 78.2% together with 12.1% second product 1b, methylisopropylketone). While the conversion of styrene oxide was 84.6% for 1 h reaction time and 99.1% for 4 h reaction time in acetone solvent, with the selectivity of 2a product (phenylacetaldehyde) was over 82%. These obtained conversions were high and comparable to the results reported in the literatures.3,4,26 It is well-known that the acid catalytic epoxide isomerization includes of the initial attack step of the electrophilic reagent to the oxirane ring, followed by the ring opening, resulting intermediate carbocations that subsequently could be transformed into aldehyde and/or ketone products.26 The branch (2-methyl-2,3-epoxybutane) and cyclic epoxide (styrene oxide) are both reactive epoxides that favours the initiation step of the isomerization reaction, and thus results the high conversion of reaction. Furthermore, core–shell magnetic ZSM-5 zeolite had a high surface area and high external surface area due to rough surface and core–shell structure. This high surface area could enhance accessibility of the epoxide molecules to the active sites of catalyst. And for the sterically hindered epoxides, the catalytic rearrangement could occur on the external surface of the zeolite.20,26 Hence the high conversion of reactants could be achieved over core–shell magnetic ZSM-5 zeolite.
Several reactions were conducted using various amount of MZZ catalyst to investigate the influence of zeolite catalyst loading on isomerization of epoxides (Fig. 2). It was found that increasing the catalyst loading from 0% to 5% w/w over reactant would increase the conversion of reactants (2-methyl-2,3-epoxybutane and styrene oxide). It is clear from the results that reaction carried out in absence of catalyst yielded a low conversion. These results showed that the contribution of the non-catalyzed reactions is negligible under the conditions used in these reactions (Fig. 2), similar to the previous reports.3,4,26 Moreover in the absence of catalyst, the selectivity of 1a and 1b product was 48.8% and 42.3% respectively, while the selectivity of 2a product was 79.1%. These results were lower than that obtained from reaction in the presence of catalyst. This indicated that the zeolite catalyst would favour the formation of aldehyde products.4,9,19 The highest conversions of reaction were obtained at catalyst loading of 5%, for which the conversion was ∼100% and 99.1% after 4 h for 2-methyl-2,3-epoxybutane and styrene oxide, respectively. At the amount of catalyst lower than 5% could still give good conversion of 2-methyl-2,3-epoxybutane and styrene oxide, but the reactions were slower and longer reaction times were required in order for them to go to completion. However, when increased the amount of catalyst from 5% to 7% w/w over reactant brought a decrease in the conversion of 2-methyl-2,3-epoxybutane as well as styrene oxide. The reduction in conversion may be due to increased formation of a polymeric material when increased catalyst loading.4 A series of experiments was therefore conducted using a zeolite catalyst loading of 5% w/w over reactant (0.05 g for 1 g reactant) for further studies.
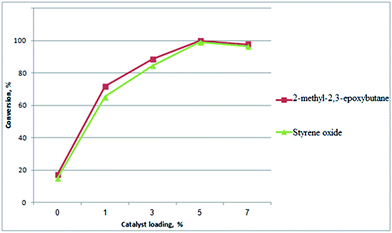 | ||
| Fig. 2 Effect of MZZ catalyst loading on isomerization of epoxide (reaction conditions: reactant (1 g) in 10 mL acetone, at 40 °C under N2 atmosphere after 2 h reaction). | ||
Another study was carried out at different temperatures, the results were shown in Table 3. The obtained results denoted that the temperature influenced significantly on the epoxide isomerizations. It could be seen that conversion of 2-methyl-2,3-epoxybutane and styrene oxide increased with the increase of temperature from 30 to 50 °C. However, when temperature further increased to 60 °C brought a decrease in the conversion of 2-methyl-2,3-epoxybutane as well as styrene oxide. This behavior could be explained that the temperature of reaction was higher than boiling point of solvent thus the evaporation of solvent could be occurred during reaction resulting in the loss of solvent and decreasing the conversion. Hence the suitable temperature for epoxide isomerization was chosen as 50 °C, and this temperature was used for next experiments.
In heterogeneous catalysis, the stability of the catalyst is an important issue from the viewpoint of a green sustainable protocol. Therefore, the recycle efficiency and stability of core–shell magnetic ZSM-5 zeolite was tested by recycling and reusing the catalyst seven times (Fig. 3). In typical, samples of magnetic ZSM-5 zeolite particles were recovered from the reactions by using external magnetic field, then were regenerated by calcination at 550 °C and reused in isomerization reactions identical to those from which the samples were recovered. The obtained results were recorded in Table 4. It was seen that the magnetic ZSM-5 zeolite catalyst could be recycled and reused efficiently for several times with only modest reduction in the conversion of 2-methyl-2,3-epoxybutane as well as styrene oxide. It indicated that the magnetic zeolite had long durability and was more resilient to catalytic deactivation. It could be assumed that the easy removing and recycling of magnetic ZSM-5 zeolite (MZZ) by applying an appropriate external magnetic field would prevent the agglomeration of the catalyst particles hence may increase the durability of the zeolite catalysts with no significant loss of catalytic activity.20,24 Moreover, the high surface area as well as high external surface area of core–shell magnetic ZSM-5 zeolite could enhance accessibility of the epoxide molecules to the active sites and enhance the diffusion and transfer of products out of catalyst pore. This could reduce the formation of coke in catalyst pore and/or surface that may limit the lifetime of catalyst and thus increase the durability of catalyst.
Furthermore, it is to be noted here that, homogeneity of core–shell catalyst particles were maintain in the samples and etching of the sacrificial SiO2 layer for resulting the voids did not continue even after several times of catalyst recycling. The shell layer of MZZ particles after several reaction cycles did not change, and small zeolite particles could not be detected in the reaction solution. The isomerization of 2-methyl-2,3-epoxybutane and styrene oxide over pristine zeolite ZSM-5, which was obtained from similar molar ratio composition of zeolite synthetic gel with MZZ particles were investigated. Under similar reaction condition, isomerization over pristine zeolite ZSM-5 resulted in conversion of 95.6% and 93.8% for 2-methyl-2,3-epoxybutane and styrene oxide, respectively. These conversion values were slightly lower than conversion values obtained from MZZ catalyst (99.4% and 98.6% for 2-methyl-2,3-epoxybutane and styrene oxide, respectively). This behavior could be attributed to the slightly higher surface area as well as higher external surface area of MZZ particles than pristine ZSM-5. This result indicated that magnetite core was well insulated from the shell layer of zeolite and did not affect the catalytic reaction. These observations strongly suggest that the reaction proceeds heterogeneously over the catalyst.
Conclusion
The catalytic activities and recycle efficiency of core–shell magnetic ZSM-5 zeolite (MZZ) was investigated. The catalytic performance of MZZ in epoxide isomerization showed that this catalyst presented a high catalytic efficiency with both high epoxide conversions and high selectivity towards the desired products. Being magnetically separable, these MZZ catalyst particles were conveniently separated, easily regenerated by heating and reused up to seven times with no significant loss of catalytic activity. This core–shell magnetically recyclable catalyst is a promising candidate for other important organic conversions and industrial catalytic applications.Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number "104.01-2013.02".References
- E. N. Jacobsen and M. H. Wu, in Comprehensive Asymmetric Catalysis II, ed. E. N. Jacobsen, A. Pfaltz and H. Yamamoto, Springer-Verlag, New York, 1999, ch. 18, pp. 649 Search PubMed.
- I. Karamé, M. L. Tommasino and M. Lemaire, Tetrahedron Lett., 2003, 44, 7687–7689 CrossRef.
- M. L. Gou, R. Wang, Q. Qiao and X. Yang, Microporous Mesoporous Mater., 2015, 206, 170–176 CrossRef CAS PubMed.
- K. Smith, G. A. El-Hiti and M. Al-Shamali, Catal. Lett., 2006, 109, 77–82 CrossRef CAS.
- B. Rickborn and R. M. Gerkin, J. Am. Chem. Soc., 1968, 90, 4193 CrossRef CAS.
- G. Stork and J. W. Schulenberg, J. Am. Chem. Soc., 1962, 84, 284 CrossRef CAS.
- M. Banerjee, U. K. Roy, P. Sinha and S. Roy, J. Organomet. Chem., 2005, 690, 1422 CrossRef CAS PubMed.
- A. M. Anderson, J. M. Blazek, P. Garg, B. J. Payne and R. S. Mohan, Tetrahedron Lett., 2000, 41, 1527 CrossRef CAS.
- D. P. Serrano, R. van Grieken, J. A. Melero and A. García, Appl. Catal., A, 2004, 269, 137–146 CrossRef CAS PubMed.
- H. Kochkar, J. M. Clacens and F. Figueras, Catal. Lett., 2002, 78, 91–94 CrossRef CAS.
- F. Zaccheria, R. Psaro, N. Ravasio, L. Sordelli and F. Santoro, Catal. Lett., 2011, 141, 587–591 CrossRef CAS.
- G. K. S. Prakash, T. Mathew, S. Krishnaraj, E. R. Marinez and G. A. Olah, Appl. Catal., A, 1999, 181, 283–288 CrossRef.
- M. Chamoumi, D. Brunel, P. Geneste, P. Moreau and J. Solofo, Stud. Surf. Sci. Catal., 1991, 59, 573–579 CrossRef CAS.
- V. V. Costa, K. A. da Silva Rocha, I. V. Kozhevnikov and E. V. Gusevskaya, Appl. Catal., A, 2010, 383, 217–220 CrossRef CAS PubMed.
- M. L. Gou, R. Wang, Q. Qiao and X. Yang, Catal. Commun., 2014, 56, 143–147 CrossRef CAS PubMed.
- R. van Grieken, D. P. Serrano, J. A. Melero and A. García, J. Catal., 2005, 236, 122–128 CrossRef CAS PubMed.
- W. F. Hölderich and H. van Bekkum, Stud. Surf. Sci. Catal., 2001, 137, 821 CrossRef.
- W. F. Hölderich, in Fine Chemicals through Heterogeneous Catalysis, ed. R. A. Sheldon and H. van Bekkum, Wiley/VCH, Weinheim, 2001, pp. 217 Search PubMed.
- R. Dimitrova, V. Minkov and N. Micheva, Appl. Catal., A, 1996, 145, 49 CrossRef CAS.
- P. H. Hoang and L. Q. Dien, Chem. Eng. J., 2015, 262, 140–145 CrossRef CAS PubMed.
- L. M. Rossi, N. J. S. Costa, F. P. Silva and R. Wojcieszak, Green Chem., 2014, 16, 2906–2933 RSC.
- C. C. Tzschucke, C. Markert, W. Bannwarth, S. Roller, A. Hebel and R. Haag, Angew. Chem., Int. Ed., 2002, 41, 3964–4000 CrossRef CAS.
- J. Svoboda, Magnetic methods for the treatment of minerals, Elsevier, 1987 Search PubMed.
- M. Shokouhimehr, Y. Piao, J. Kim, Y. Jang and T. Hyeon, Angew. Chem., 2007, 119, 7169–7173 CrossRef PubMed.
- R. van Grieken, D. P. Serrano, J. A. Melero and A. García, J. Mol. Catal. A: Chem., 2004, 222, 167–174 CrossRef CAS PubMed.
- D. P. Serrano, R. van Griekena, J. A. Melero, A. Garcí and C. Vargasa, J. Mol. Catal. A: Chem., 2010, 318, 68–74 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |