Surface-initiated polymerization of A–A/B–B type conjugated monomers by palladium-catalyzed Stille polycondensation: towards low band gap polymer brushes†
Hussein Awada,
Antoine Bousquet,
Christine Dagron-Lartigau* and
Laurent Billon*
IPREM CNRS-UMR 5254, Equipe de Physique et Chimie des Polymères, Université de Pau et des Pays de l’Adour, Hélioparc, 2 avenue Président Angot, 64053 Pau Cedex 9, France. E-mail: laurent.billon@univ-pau.fr; christine.dagron-lartigau@univ-pau.fr
First published on 1st September 2015
Abstract
A surface-initiated polycondensation which enables access to well-defined core–shell nanoparticles is described. Stille polymerization was initiated from the surface of palladium catalyst-immobilized zinc oxide nanorods. For the first time, a low band gap polymer, poly[(4,4′-bis(2-ethylhexyl)dithieno-[3,2-b:2′,3′-d]silole)-2,6-diyl-alt-(2,1,3-benzothiadiazole)-4,7-diyl] (PSBTBT), was anchored on zinc oxide nanorods to create hybrid materials with tunable photophysical properties.
Ever since it was discovered that strong and flexible films of polyacetylene could be prepared by a Ziegler–Natta polymerization, numerous conjugated polymers have been synthesized for various applications, for example light-emitting diodes, sensors or photovoltaics.1 The promise of organic-based devices lies in their low cost, light weight, conformability, flexibility and versatility due to the wide potential of chemical structures. At first, the objectives in the conjugated polymer chemistry field were to conceive new materials with improved control of electrical and optical properties and improved processibility; in particular to synthesize soluble conjugated polymers (CPs). A recent breakthrough in this field was the use of the so-called “low band gap” (LBG) polymers, which are able to harvest more photons than the “classical” polymers, such as CPs and poly(3-hexylthiophene) (P3HT).2 These are often copolymers with alternated electron rich (donor) and electron deficient (acceptor) monomer units in their microstructure. The high energy level for the HOMO of the donor unit and the low energy level for the LUMO of the acceptor unit decrease the band gap due to an intra-chain charge transfer. Since the band gap of these polymers is lower than 1.8 eV, photons of wavelengths above 600 nm are absorbed. Thus photovoltaic efficiencies up to 10.8% have been reached with these new macromolecules in bulk heterojunctions with fullerene derivatives.3–6
In parallel, a few research groups turned their attention recently toward the possibility of linking conjugated polymers to inorganic, metal or carbon-based surfaces, by covalently binding the two components in order to design new hybrid materials.7 Kiriy’s and Locklin’s research groups developed the so-called “surface-initiated Kumada catalyst transfer polycondensation” (SI-KCTP) to create P3HT brushes from different substrates such as silica particles (core@shell, SiO2@P3HT), silicon, indium tin oxide or gold wafers.8–11 Luscombe and colleagues developed SI-KCTP for the polymerization of 3-methylthiophene from an ITO wafer functionalized with an aryl-nickel-bromide initiator.12 Very recently Bielawski’s group extended the scope of surface-initiated polymerization of a conjugated monomer by performing the polycondensation of a phenylene–ethynilene A–B type monomer in a chain-growth manner.13 Another methodology, called “grafting through” is based on the anchoring of a polymerizable group (most of the time a monomer) on the substrate. Polymer chain initiation takes place in solution or in bulk and during the propagation step; the growing chains react with the functional group attached to the surface and then further propagate with the free monomers. This methodology has been applied for the grafting of conjugated macromolecules such as polyfluorene on quartz wafer via Yamamoto coupling polymerization14 or poly(phenylene vinylene) on CdSe quantum dots via Heck coupling polycondensation.15
For the moment, only a few conjugated macromolecules have been initiated from a surface and many synthetic efforts are still needed to find ways to covalently anchor CPs to a wide variety of surfaces (metal, metal oxide, etc.). In this communication, we combine two very promising fields by reporting the creation of low band gap polymer brushes. The surface initiation of an (A–A/B–B) type step growth polymerization has been performed and zinc oxide@LBG nanoparticles were created for the first time.
In this report, poly[(4,4′-bis(2-ethylhexyl)dithieno-[3,2-b:2′,3′-d]silole)-2,6-diyl-alt-(2,1,3-benzothiadiazole)-4,7-diyl] (PSBTBT) was synthesized by the polymerization of 4,7-dibromo-2,1,3-benzothiadiazole (M1) and 4,4′-bis(2-ethyl-hexyl)-5,5′-bis(trimethyltin)-dithieno[3,2-b:2′,3′-d]silole (M2). This polymer was chosen for its broad absorption, high hole mobility, good photochemical stability and high power conversion efficiency of 5.1%.16 The monomers were obtained with high purity (97% for M1 and 99% for M2, synthesis and characterization in the ESI†). They were first copolymerized in a chlorobenzene solution using Stille polycondensation. 1H and 13C nuclear magnetic resonance (1H/13C NMR) showed all the characteristic peaks of the polymer, and UV-visible spectroscopy was used to evaluate the optical band gap as 1.52 eV (estimated from the low energy band edge of the optical spectrum, Eg = 1240/λonset, Fig. SI4†). Although the molar mass could not be measured by gel permeation chromatography (polymers insoluble in THF), comparison of the absorption spectra with the literature confirmed that the degree of polymerization is high enough to reach the maximum conjugation length.17
Once the experimental conditions were successful for solution polymerization, they were applied for surface-initiated polymerization by functionalizing ZnO nanorods (length = 150 nm, ø = 30 nm and specific surface area = 24 m2 g−1) with initiating sites at the surface (Scheme 1). For this purpose [2-((4-bromo-phenyl)-ethyl)]-triethoxysilane (Si-PhBr) was synthesized (see ESI†) and grafted onto ZnO nanorods. In the second step the palladium catalyst (Pd2(dba)3, tris(dibenzylideneacetone)dipalladium(0)) was anchored to the substrate by the creation of a phenyl-Pd(dBa)2–Br complex. After each step, the treated particles were purified from unreacted Si-PhBr or Pd(dBa)2 and byproducts by repeated dispersion/centrifugation cycles. ZnO nanoparticles were chosen for their quality as electron acceptors making them of great use in hybrid solar cells.12 Part of the reason for the low efficiency of hybrid photovoltaics18 is related to the properties of the ZnO/polymer interface. Because excitons formed in a polymer layer need to dissociate at the interface, contaminants that might exist at this interface will be very harmful. Therefore it is essential for an electron donor and an electron acceptor to be placed in direct contact for the efficient dissociation of excitons.19
The success of this grafting procedure was proven by X-ray photoelectron spectroscopy (XPS); Table 1 gives an overview of the surface chemical composition. When ZnO nanorods were functionalized with Si-PhBr, the zinc surface content decreased, the carbon content increased, and finally bromine and silicon appeared. From a quantitative point of view, thermogravimetric analysis (TGA) (Fig. SI8†) of ZnO and ZnO@PhBr showed an organic mass loss of 1.5% which corresponded to a phenylbromide grafting density of 2 molecules per nm2. The aforementioned value was consistent with literature reports.13,20 Having proved the bromobenzene surface coverage of the ZnO nanorods, subsequent efforts were directed towards the anchoring of the palladium catalyst on the substrate. ZnO@PhBr nanorods were then reacted with an excess of Pd2(dba)3 (calculated from the specific surface area and the grafting density) in tetrahydrofuran (THF) at 50 °C for 6 h. The color of the particles turned from white to dark grey. XPS analysis of ZnO@PhPdBr showed a slight increase in the carbon content due to the dba ligands and the appearance of a peak at 335 eV pertaining to the palladium (Pd) element.
| Substrate/atom binding energy (eV) | C 285 | O 530.5 | Zn 1022.5 | Br 70.1 | Si–O 102.8 | Si–C 101.1 | Pd 335.4 | S 164 | Sn 487.4 | N 399.4 | w.l.a % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a w.l.: weight loss percentage determined by thermogravimetric analysis. | |||||||||||
| ZnO | 19.4 | 45.6 | 35.0 | — | — | — | — | — | — | — | 2.7 |
| ZnO@PhBr | 20.3 | 46.3 | 28.3 | 1.5 | 3.7 | — | — | — | — | — | 4.1 |
| ZnO@PhPdBr | 22.6 | 43.9 | 28.4 | 1.3 | 2.2 | — | 1.6 | — | — | — | — |
| ZnO@PSBTBT 2 | 30.9 | 37.2 | 23.1 | 1.7 | 2.8 | 0.5 | 1 | 1.1 | 0.7 | 1.2 | 6.3 |
| ZnO@PSBTBT 4 | 49.1 | 26.4 | 12.9 | 2.2 | 1.2 | 1 | 1 | 3.12 | 1.2 | 2.1 | 7.4 |
| ZnO@PSBTBT 6 | 57 | 21.2 | 9.4 | 0.8 | 1.7 | 1.6 | 0.7 | 4.6 | 0.7 | 2.9 | 13.3 |
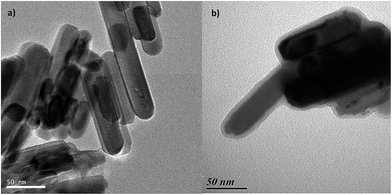
The surface polymerization was then performed using these ZnO@PhPdBr nanorods in a chlorobenzene solution of monomers M1 and M2 at 150 °C for 2, 4 and 6 h to obtain particles ZnO@PSBTBT 2, 4 and 6, respectively. During the reaction, the dark brown solution turned first dark blue and then greenish, which is a clear sign of polymerization leading to conjugation length extension. The particles were then submitted to dispersion/centrifugation cycles several times, until the supernatant became colorless and its UV-visible spectrum was featureless. By this procedure, a consistent amount of “free” polymer (named PSBTBT 2, 4 and 6, respectively) was extracted from the core@shell particles. The fact that polymerization happened also in solution is inherent to the reaction process. In the present case, the polymerization was initiated from the particles since the catalyst was attached to their surface. Once a stannane monomer M2 reacts with ZnO@PhPdBr, the palladium catalyst is released from the surface to the solution and polymerization starts by activation of the brominated monomer M1. With this polymerization being a step-growth reaction, the solution macromolecules may also couple to the substrate organic sites. The ratio between the free and grafted chains was 90/10 (in weight). This ratio could be decreased by decreasing the quantity of monomer in the initial solution. We instead kept the experimental conditions the same to obtain both the free chains that will be used as a matrix in further solar cell applications, and the grafted particles, in “one pot”. The “free” polymer chains were characterized by gel permeation chromatography (Fig. SI19†) but their insolubility in THF led to an under-estimation of their molar masses and dispersity. Instead, UV-visible spectroscopy was performed to show an increase of the absorption maximum wavelength with increasing polymerization time. Again the maximum conjugation length was obtained for PSBTBT 4 and 6 (Fig. 1),21 with an optical band gap of 1.51 eV and an electronic band gap of 1.58 eV (determined by cyclic voltammetry, see Fig. SI21–23†). 1H and 13C NMR results showed all the characteristic peaks of the polymers (Fig. SI24–26†).
 | ||
| Fig. 1 UV-visible absorption spectra of free PSBTBT in chloroform obtained after 2, 4 and 6 h of reaction. | ||
At the end of the washing procedure the particles were dark blue/green. XPS characterization was also performed and proved the presence of the polymer at the surface with an increase of the carbon and silicon atom content and a decrease of zinc and oxygen. Moreover there was an appearance of three new elements namely sulfur, silicon (Si–C) and nitrogen pertaining to the monomer unit and in a quantity which increased with polymerization time. Interestingly, the presence of bromine, palladium and tin indicates the presence of active chains, indicating the possibility of extending the length of the polymer brush.
Another indication of successful grafting was brought by the silicon binding energy which was divided into two populations: at 102.3 eV for Si–O (the silane anchoring group) and at 100.7 eV for Si–C (the monomer unit). Deconvolution of the Si signal at 103–101 eV showed an increase in the ratio of the Si–C/Si–O signal areas (see Fig. SI12†) with polymerization time. This is also consistent with TGA analysis of the grafted particles revealing an increase in the weight loss percentage with polymerization time (last column of Table 1, Fig. SI13†).
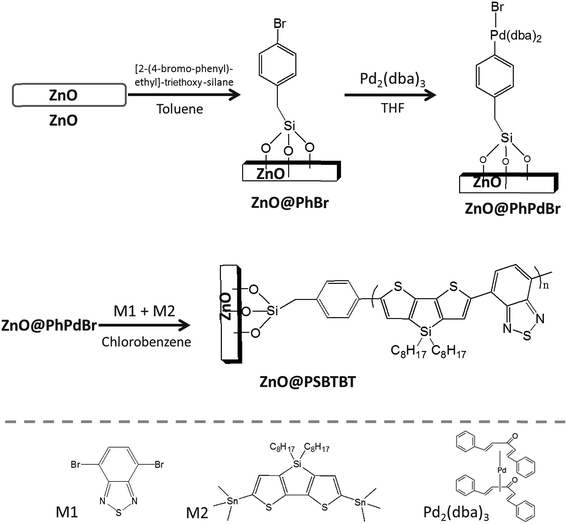
Transmission electron microscopy (TEM) was used to evaluate the thickness of the grafted LBG layer on the surface of the ZnO NRs. Fig. 2 shows both bare particles and ZnO@PSBTBT 2. A clear, complete and homogeneous polymer shell was identified around the ZnO NRs after grafting, leading to a core@shell hybrid material. The average polymer shell thickness (h) as measured from the images for the different hybrid nanorods was 5 ± 1 nm.
TEM analysis of ZnO@PSBTBT 4 and 6 also emphasized this homogeneous layer (see Fig. SI15–17†) but no clear increase in the shell thickness was detected. Our hypothesis is that this can be explained by considering the mechanism of surface-initiated step growth polymerization. During the reaction, monomers and multimers in the solution can couple with the surface. If long multimers anchor onto the surface then a steric hindrance is created, some short surface chains are hidden and their propagation is then limited. This phenomenon is exacerbated with polymerization time since the steric hindrance of the chains grows. Therefore, the ability for long chains to reach active sites at the surface decreases and the dispersity of the grafted chains increases. As a final consequence the long anchored macromolecules have space to fold over the surface, reducing the apparent thickness of the organic shell. A limitation of this methodology is demonstrated in Fig. SI18† in which crosslinking of the nanoparticles by the polymer happened as the conversion or the particle/monomer concentration was too high.
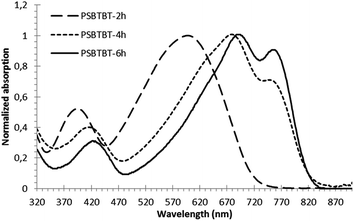
It is interesting to observe that the photophysical properties of the LGB polymer brushes can significantly differ from those of the same polymer in solution. Fig. 3 presents the superimposition of the UV-visible absorbance spectra in chloroform of the grafted ZnO@PSBTBT 2 nanorods, a mixture of bare ZnO nanoparticles/free polymer 2 and finally the free polymer 2 for comparison. The first feature is the presence in the UV region at 370 nm of the ZnO absorption band. Free polymer chains exhibit a maximum absorption at a wavelength of 600 nm, which was the same in the case of the mixture, indicating that the macromolecules were well solvated in CHCl3 and behaved independently. On the contrary, upon grafting, the maximum absorbance was drastically red-shifted to a wavelength of 680 nm with a clear shoulder at 750 nm.
 | ||
| Fig. 3 UV-visible spectra of the ZnO@PSBTBT 2 (bold line), ZnO + PSBTBT 2 (dash) and PSBTBT 2 (line) in chloroform solution. | ||
This bathochromic shift, i.e. red shift of 70–80 nm, nicely reflects a significant planarization of the polymer backbone and high inter-chain interaction resulting in efficient delocalization of the π-conjugated electrons. This behaviour is related to the high grafting density of the polymer brushes which forces the macromolecules to be in close contact and packed. Kiriy et al. have already observed the same effect for P3HT brushes created on silica particles via the “grafting from” methodology.10 On the contrary, when the grafting density is lower, like in the case of the “grafting onto” methodology, the anchored polymer is swollen and its maximum absorption remains the same as the corresponding free polymer.22,23 As a comparison, thin films of the free polymer were obtained by spin-coating and annealed, and their UV-visible spectra presented the same features as those of the polymer brushes (Fig. SI10†). The UV-visible absorbance was also recorded for ZnO@PSBTBT 4 and 6 and showed a high degree of order and packing in the polymer brushes, with a strong increase of the intensity of the vibronic structures peak at 780–800 nm (Fig. SI9†).
To conclude, the covalent attachment of a low band gap conjugated polymer on an inorganic metal oxide substrate was demonstrated for the first time. Stille polycondensation was successfully initiated from the surface of palladium catalyst-immobilized zinc oxide nanorods. Due to the inherent step growth mechanism of the polymerization, the synthesized polymer was both grafted onto the ZnO surfaces and obtained as free macromolecules. XPS and TEM analysis demonstrated that the macromolecules anchored on the nanorods formed a homogeneous organic shell of 5 nm thick. UV-visible spectroscopy exhibited a strong bathochromic effect of 70–80 nm and relevant vibronic structures because of the high grafting density and strong π–π stacking which tend to closely pack the confined polymer chains grafted at the surface. This hybrid ZnO@LBG, as an acceptor@donor material, is the first step toward our target to obtain “patchy” particles with a ZnO core partially covered with a low band gap polymer in order to maintain electron percolation between particles.
Acknowledgements
This work was supported by the French Ministry of Research and COST Action MP1202 “Rational design of hybrid organic–inorganic interfaces: the next step towards advanced functional materials”. The authors thank Dr D. Flahaut for XPS analyses and Dr M. H. Delville for providing ZnO nanoparticles.References
- A. G. MacDiarmid, Angew. Chem., Int. Ed., 2001, 40, 2581–2590 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2007, 91, 954–985 CrossRef CAS PubMed.
- A. Pron, P. Berrouard and M. Leclerc, Macromol. Chem. Phys., 2013, 214, 7–16 CrossRef CAS PubMed.
- X. Guo, N. Zhou, S. J. Lou, J. Smith, D. B. Tice, J. W. Hennek, R. P. Ortiz, J. T. L. Navarrete, S. Li, J. Strzalka, L. X. Chen, R. P. H. Chang, A. Facchetti and T. J. Marks, Nat. Photonics, 2013, 7, 825–833 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- A. Bousquet, H. Awada, R. C. Hiorns, C. Dagron-Lartigau and L. Billon, Prog. Polym. Sci., 2014, 39, 1847–1877 CrossRef CAS PubMed.
- L. Yang, S. K. Sontag, T. W. Lajoie, W. Li, N. E. Huddleston, J. Locklin and W. You, ACS Appl. Mater. Interfaces, 2012, 4, 5069–5073 CAS.
- S. K. Sontag, N. Marshall and J. Locklin, Chem. Commun., 2009, 3354–3356 RSC.
- V. Senkovskyy, R. Tkachov, T. Beryozkina, H. Komber, U. Oertel, M. Horecha, V. Bocharova, M. Stamm, S. A. Gevorgyan, F. C. Krebs and A. Kiriy, J. Am. Chem. Soc., 2009, 131, 16445–16453 CrossRef CAS PubMed.
- V. Senkovskyy, N. Khanduyeva, H. Komber, U. Oertel, M. Stamm, D. Kuckling and A. Kiriy, J. Am. Chem. Soc., 2007, 129, 6626–6632 CrossRef CAS PubMed.
- N. Doubina, J. L. Jenkins, S. A. Paniagua, K. A. Mazzio, G. A. MacDonald, A. K. Y. Jen, N. R. Armstrong, S. R. Marder and C. K. Luscombe, Langmuir, 2012, 28, 1900–1908 CrossRef CAS PubMed.
- S. Kang, R. J. Ono and C. W. Bielawski, J. Am. Chem. Soc., 2013, 135, 4984–4987 CrossRef CAS PubMed.
- S. B. Jhaveri, J. J. Peterson and K. R. Carter, Langmuir, 2009, 25, 9552–9556 CrossRef CAS PubMed.
- H. Skaff, K. Sill and T. Emrick, J. Am. Chem. Soc., 2004, 126, 11322–11325 CrossRef CAS PubMed.
- J. Hou, H. Y. Chen, S. Zhang, G. Li and Y. Yang, J. Am. Chem. Soc., 2008, 130, 16144–16145 CrossRef CAS PubMed.
- W. J. E. Beek, M. M. Wienk and R. A. J. Janssen, Adv. Funct. Mater., 2006, 16, 1112–1116 CrossRef CAS PubMed.
- L. Whittaker-Brooks, W. E. McClain, J. Schwartz and Y. L. Loo, Adv. Energy Mater., 2014, 4 DOI:10.1002/aenm.201400585.
- C. Leow, T. Ohnishi and M. Matsumura, RSC Adv., 2015, 5, 6232–6237 RSC.
- S. K. Sontag, G. R. Sheppard, N. M. Usselman, N. Marshall and J. Locklin, Langmuir, 2011, 27, 12033–12041 CrossRef CAS PubMed.
- M. C. Scharber, M. Koppe, J. Gao, F. Cordella, M. A. Loi, P. Denk, M. Morana, H. J. Egelhaaf, K. Forberich, G. Dennler, R. Gaudiana, D. Waller, Z. Zhu, X. Shi and C. J. Brabec, Adv. Mater., 2010, 22, 367–370 CrossRef CAS PubMed.
- H. Awada, H. Medlej, S. Blanc, M. H. Delville, R. C. Hiorns, A. Bousquet, C. Dagron-Lartigau and L. Billon, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 30–38 CrossRef CAS PubMed.
- J. Xu, J. Wang, M. Mitchell, P. Mukherjee, M. Jeffries-El, J. W. Petrich and Z. Lin, J. Am. Chem. Soc., 2007, 129, 12828–12833 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional figures such as TEM images, thermogravimetric analysis and UV spectra. See DOI: 10.1039/c5ra08027d |
| This journal is © The Royal Society of Chemistry 2015 |