Isolation of circulating tumour cells by physical means in a microfluidic device: a review
Abstract
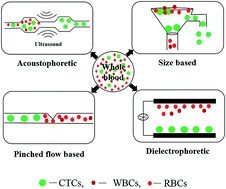
Isolation and enumeration of circulating tumour cells (CTCs) from human blood has a huge significance in diagnosis and prognosis of cancer. Utilization of the unique microscale flow phenomena called microfluidics offers ability to efficiently isolate CTCs from other haematological cells. The improvement in microfluidic technology allows time and cost efficient isolation of CTCs in a continuous manner utilising only up to nano- or micro-litres samples. This technology could potentially lead to the fabrication of cheap, disposable and transparent devices for sorting and molecular examination of even single cell. Additionally, the potential to physically entrap and capture rare CTCs in microfluidic devices can eliminate the need of expensive antibodies normally used for immune capturing of these rare cells which would further reduce the cost of operation. During the last few years, several innovative and intricate microfluidic designs to isolate and capture these extremely rare cells from the whole blood samples without using specific antibodies have been published. Herein, we review the recent literature on exploiting physical characteristics of tumour cells to efficiently isolate them from billion other cells and discuss the intricate design perspective of microfluidic devices for efficient in vitro cancer diagnosis and prognosis.

 Please wait while we load your content...
Please wait while we load your content...