A novel non-releasing antibacterial poly(styrene-acrylate)/waterborne polyurethane composite containing gemini quaternary ammonium salt
Abstract
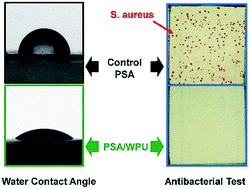
A series of novel non-releasing antibacterial polymer coatings, incorporating gemini quaternary ammonium salt modified waterborne polyurethane into a commercial poly(styrene-acrylate), were designed and prepared via a facile blending strategy. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy and differential scanning calorimetry (DSC) results are used to prove the compatibility between the polyurethanes and poly(styrene-acrylate)s. X-ray photoelectron spectroscopy (XPS) and water contact angle (WCA) measurements are used to clarify the surface structure and properties of polymer coatings, indicating gemini quaternary ammonium salts (GQAS) attached onto waterborne polyurethane chains could migrate and aggregate onto surfaces of these polymer blending coatings. On the basis of the antibacterial characteristics of GQAS, these polymer blending coatings showed high efficiency in killing airborne bacteria, e.g. S. aureus and E. coli, in contact-killing tests. Thus, the antibacterial coatings and blending strategy are promising for the development of environmentally friendly materials.

 Please wait while we load your content...
Please wait while we load your content...