Synthesis of 3-dimensional porous graphene nanosheets using electron cyclotron resonance plasma enhanced chemical vapour deposition
Rajesh Thomas* and
G. Mohan Rao
Dept. of Instrumentation and Applied Physics, Indian Institute of Science, Bangalore, India. E-mail: thomasphy@gmail.com
First published on 17th September 2015
Abstract
Microwave plasma driven chemical vapour deposition was used to synthesize graphene nanosheets from a mixture of acetylene and hydrogen gas molecules. In this plasma, acetylene decomposes to carbon atoms that form nanostructures in the outlet plasma stream and get deposited on the substrate. The GNS consists of a few layers of graphene aligned vertically to the substrate. Graphene layers have been confirmed by high-resolution transmission electron microscopy, and Raman spectral studies were conducted to observe the defective nature of the sample. The growth of nanosheets in a vertical direction is assumed to be due to the effect of electric field and from the difference in the deposition rate in the axial and parallel directions. These vertical graphene sheets are attractive for various applications in energy storage and sensors.
1. Introduction
Carbon materials have received considerable attention in recent years due to their unique properties in various forms such as diamond, graphite, graphene, amorphous carbon, fullerenes and nanotubes. These structures have shown remarkable differences in properties and hence in potential applications, such as photovoltaics,1–4 field emission,5 energy storage,6 transistors,7 biology,8 and hydrogen storage,9 due to their peculiar chemical, electrical, optical and mechanical properties.10There are several methods for synthesizing graphene, which can be broadly classified as top down and bottom up approaches. In bottom up approach, graphene is synthesized from atomic or molecular species, whose precursor particles are allowed to gradually grow in size. In top down synthesis, graphite, carbon fibers and carbon nanotubes are the starting materials and individual graphene layers are extracted or peeled off either by physical, electrochemical or chemical methods.11–14 The road block in making graphene devices is the absence of a viable method for mass production. Chemical vapour deposition (CVD),15 mechanical exfoliation,16 chemical exfoliation,17 liquid phase exfoliation18 and epitaxial growth19 generally produce single, bi-layer or tri-layer graphene sheets, but have low yield and thus are unsuitable for mass production. Recently, graphene has been synthesized from chemical routes,20 in which graphite oxide readily exfoliates in water and the suspension can be chemically reduced to yield graphene. This highly reproducible chemical route is among the first techniques to promise the mass production of graphene, but the synthesis of graphene through chemical routes, which involves many toxic chemicals, is not compatible with the present microelectronic device technology.
Vertically oriented graphene (VG) nanosheets are a special type of two dimensional nanostructures that are oriented vertically on a substrate. These VG nanosheets are highly porous and consist of a few layers of graphene with a layer number of 1–15 and the pore diameter is in the range of 50–200 nm. These carbon nanostructures have unique features such as sharp edges, vertical morphology, high surface to volume ratio and can be found in a great number of applications, including energy storage (Li ion batteries21,22 and supercapacitors), gas sensors, field emitters, biosensors and fuel cells.1–10
Microwave plasma enhanced chemical vapour deposition (MW-PECVD) has been established as a key method for producing graphene nanosheets. PECVD technique has many advantages over thermal CVD, including low substrate temperature, higher growth selectivity, better control in nanostructure ordering,23 and low operating pressure (10−3 to 10−4 mbar), leading to high purity films, as well as high ionization coefficient, that enabling a higher growth rate.24–27
2. Experimental
Graphene nanosheets were synthesized in a home-made ECR-PECVD set-up (Fig. 1). The ECR system consisted of a plasma source chamber mounted on a stainless steel chamber. Beam diameter at the exit of the source was about 100 mm. A flexible substrate holder allowed the free positioning of the substrate along the axis of the source chamber. The substrate was kept at a distance of 10 cm from the exit of the plasma source and the ion density at the surface of the substrate was measured to be approximately 9.5 × 1010 cm−3. The details of the experimental system and working principle have been described in our group's previous study.28Annealed polycrystalline copper (Cu) foil with a thickness of 0.25 mm (Alfa Aesar) and an area of 1 cm2 was used as the substrate. The substrate was cleaned with dilute nitric acid and rinsed with de-ionized (DI) water. Prior to the deposition, the substrate was heated at 500 °C for 30 min. Subsequently, the substrate was treated in H2 plasma for 10 min at 500 °C. The carbon deposition was carried out at 700 °C for 30 min. A mixture of hydrogen (H2) and acetylene (C2H2) was used at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Plasma power was set to 500 W and the working pressure was set at 7 × 10−4 mbar. Furthermore, GNS was deposited over different substrates such as platinum or nickel coated copper, SiC, SiO2 and quartz.
1. Plasma power was set to 500 W and the working pressure was set at 7 × 10−4 mbar. Furthermore, GNS was deposited over different substrates such as platinum or nickel coated copper, SiC, SiO2 and quartz.
3. Results and discussion
3.1 Growth of graphene nanosheets
Several reports have been published for the synthesis of graphene nanosheets. Wu et al.25 and Zheng et al.23 described the synthesis of graphitic carbon nanostructures composed of a few layers of graphene vertically standing on a substrate via microwave plasma enhanced chemical vapor deposition (MW-PECVD). Chen et al. reported the GNS synthesis using direct current (dc) PECVD at atmospheric pressure on various substrates.29–31 The exact mechanism of the GNS growth is not known completely.The vertical nature of GNS may be due to the difference of growth in horizontal to vertical directions. The exact mechanism of GNS growth is not well understood, and it is assumed that electric field plays an important role.32,33 It has also been reported that electric fields play an important role in the alignment of CNTs in microwave plasmas.34,35 The electric field tends to align vertically to the plate, when metallic objects are directly placed in the plasma field even without any biasing. In our experiment, we placed the substrate over a metallic plate from where the electric field can influence the GNS growth. In addition to the electric field, the vertical characteristic was also affected by H2 dilution, which also contributed to the shaping of the nanowall morphology by etching the loosely bonded carbon atoms and amorphous carbon. Thus the graphitization of graphene nanowall could be enhanced.
From the SEM observations, it was found that an amorphous carbon film was deposited on the Cu substrates from the acetylene plasma. At a certain temperature, in the presence of plasma, the amorphous carbon film was bombarded to form random small size nanoflakes. These nanoflakes act as the nucleation sites. Subsequently, the GNS grew on the nucleation positions and aligned vertically on the substrate. The vertical nature of GNS is due to the difference in growth rates between the parallel and perpendicular directions to the graphene nanosheet.36 The local electric field from the plasma also has an effect on the vertical orientation of GNS. The electric-field dependent orientation has been observed for carbon nanotubes and nanowalls.37,38 Herein, we study the growth of GNS films by various growth parameters and discuss how the parameters, such as deposition time, flow rate of acetylene, temperature and microwave power, influence the growth. Hydrogen dilution with acetylene plasma also has an influence on the growth of GNS.
Various parameters influence the growth of graphene sheets for the plasma CVD deposition. The surface morphology of GNS over Cu substrate at different deposition time is given in Fig. 2. All the depositions were carried out at a microwave power of 500 W and at a substrate temperature of 700 °C. The flow rate of acetylene was set to 12.4 sccm and the ratio of C2H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 was 1
H2 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. After one min of deposition, no GNS nucleation was observed (Fig. 2a). An amorphous kind of carbon was observed after 3 min of deposition (Fig. 2b). Then, uniform nucleation of GNS started after 5 min of deposition (Fig. 2c). From SEM observations, it was found that the nucleation started after a certain period of deposition time elapsed. The thickness of the GNS film was measured about 200 nm (Fig. 2d).
2. After one min of deposition, no GNS nucleation was observed (Fig. 2a). An amorphous kind of carbon was observed after 3 min of deposition (Fig. 2b). Then, uniform nucleation of GNS started after 5 min of deposition (Fig. 2c). From SEM observations, it was found that the nucleation started after a certain period of deposition time elapsed. The thickness of the GNS film was measured about 200 nm (Fig. 2d).
Fig. 3 shows the SEM images of GNS grown by varying the flow rate of C2H2. All the depositions were carried out at a microwave power of 500 W, substrate temperature of 700 °C and deposition time of 5 min. At the flow rate of 5 sccm of C2H2, an amorphous carbon was deposited on the substrate and the GNS formation was not visible (Fig. 3a). When the flow rate was increased to 12 sccm, nucleation of GNS started (Fig. 3b) and at 25 sccm, there was good GNS formation on the substrate (Fig. 3c). The GNS was highly porous and uniformly distributed. The mixture of C2H2 and H2 has been taken as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. There should be a minimum concentration of C2H2 required to obtain uniform GNS over the entire substrate.
2. There should be a minimum concentration of C2H2 required to obtain uniform GNS over the entire substrate.
The SEM images (Fig. 3d–f) show the effect of microwave power on the growth of GNS. The microwave power was varied from 100 W to 500 W. For the deposition of GNS, the flow rate of C2H2 was kept constant at 12 sccm. All the deposition was carried out at a substrate temperature of 700 °C and 5 min of deposition time. Furthermore, at the microwave power of 100 W (Fig. 3d), the GNS film contains mainly amorphous carbon. When the microwave power was increased to 300 W, the nucleation of GNS started (Fig. 3e) and at 500 W, well defined GNS growth was observed (Fig. 3f). The growth of GNS at different temperatures was studied at a constant C2H2 flow rate of 12 sccm, microwave power of 500 W and deposition time of 5 min. When deposition was carried out at room temperature, the film contained irregular and amorphous carbon, as shown in Fig. 3g. When the temperature was increased to 400 °C and 700 °C, uniform distribution and initiation of GNS nucleation could be observed (Fig. 3h and i).
Nonomura et al. studied the effect of H2 dilution on GNS growth by hot wire CVD. It was found that GNS growth varies with different hydrogen dilution with methane (CH4). The surface morphology of GNS changes at different hydrogen concentrations. One of the effects of H2 dilution would be the etching activity of hydrogen radical. Hydrogen radical etches loosely bonded carbon and enhance the graphitization of diamond-like carbon (DLC) film.39,40 Hydrogen radicals etch away disordered carbon atoms such as amorphous carbon rather than graphitic carbon. The bond strength of disordered carbon is weaker than that of ordered carbon.
Fig. 4a shows the SEM image of GNS film without hydrogen. A lot of amorphous carbon was deposited on Cu substrate and the thickness of the graphene sheet increased. Amorphous carbon was present in the film even after equal rates of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) H2 and C2H2 was used for deposition (Fig. 4b). Highly porous and uniformly distributed GNS were observed for the films that used 2
1) H2 and C2H2 was used for deposition (Fig. 4b). Highly porous and uniformly distributed GNS were observed for the films that used 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of hydrogen and acetylene (Fig. 4c). The amorphous carbon was etched out due to the presence of more hydrogen radicals in the plasma. If the ratio of hydrogen is increased, more carbon will be etched out and the porous cavity will be increased (Fig. 4d).
1 ratios of hydrogen and acetylene (Fig. 4c). The amorphous carbon was etched out due to the presence of more hydrogen radicals in the plasma. If the ratio of hydrogen is increased, more carbon will be etched out and the porous cavity will be increased (Fig. 4d).
3.2 Morphology of GNS over different substrates
GNS films were deposited over different substrates using plasma enhanced CVD technique. The substrates used for the deposition were SiO2, quartz, amorphous SiC (a-SiC), Cu, Pt and Ni. Pt and Ni were sputtered over the Cu substrate. The average thickness of the coating was ∼40 nm. An amorphous SiC coating was synthesised with reactive sputtering of silicon with acetylene (C2H4) over Si substrate and was then used as the substrate. All the substrates were cleaned with deionized (DI) water and ultrasonicated for 10 min. Furthermore, the substrates were dried with dry air and all the GNS deposition was carried out at a microwave power of 500 W, C2H2 flow rate of 12 sccm, substrate temperature of 700 °C and deposition time of 25 min. The ratio of H2 and C2H2 mixture was about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The morphology of the as-deposited GNS over metallic substrates, such as Cu, Pt and Ni, was observed by scanning electron microscopy (Fig. 5). The SEM images show a highly porous and vertically aligned thin wall type of morphology for the GNS electrode, which has a large surface to volume ratio. The morphology of NiGNS and PtGNS clearly reveals that the spherical flower-like graphene petals with a diameter of 1–2 μm were uniformly distributed over the surface of the substrate. The graphene walls formed over Ni and Pt coated Cu substrates are highly porous and branched (Fig. 5c–f), whereas GNSs over bare Cu substrate (Fig. 5a and b) are straight with a pore size of 50–150 nm in diameter.
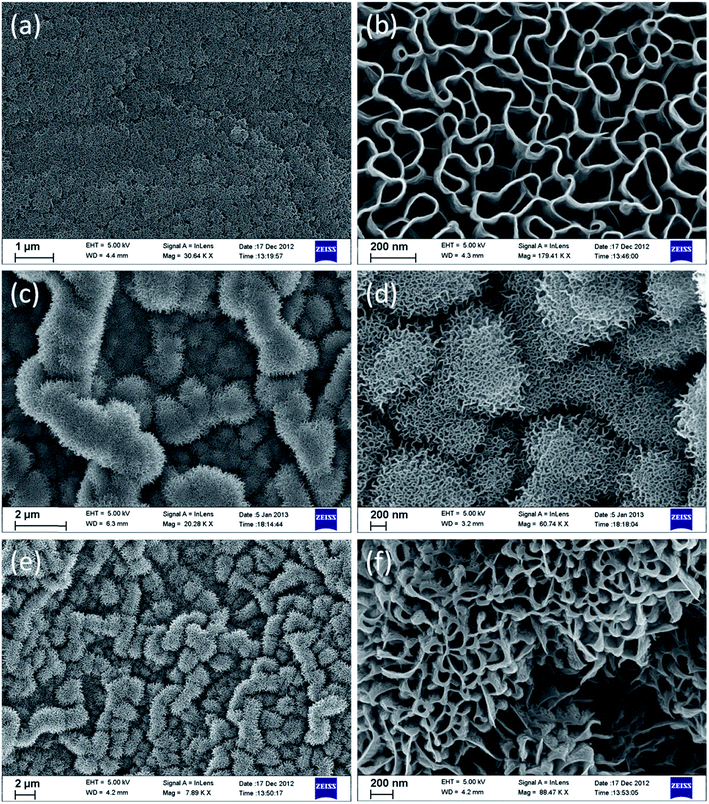 | ||
| Fig. 5 Scanning electron microscopy (SEM) images of graphene nanosheets on (a and b) copper, (c and d) nickel and (e and f) platinum substrates. | ||
Fig. 6 shows the SEM image of GNS over insulating substrates such as SiO2 (Fig. 6a and b), quartz (Fig. 6c and d) and a-SiC (Fig. 6e and f). The density of GNS over these substrates varies. The edges of the vertically grown GNS are highly curled and spiralled. From the SEM images, it can be observed that the nature of the substrate has an effect on GNS growth. High density GNS films were grown over the metallic substrates such as Cu, Pt and Ni. On the other hand, the growth of GNS on insulating substrates was less dense. The depositions of GNS over these substrates were performed after etching the surface in H2 plasma for 10 min. While etching, the surface of Pt and Ni coated Cu substrates became very rough. The high density of GNS over Pt and Ni coated substrates may be due to the effect of roughness.
The cross section views of CuGNS and PtGNS are shown in Fig. 7a and b. The highly branched morphology of NiGNS and PtGNS offers high surface area and high ratio of porosity. The HRTEM image further shows that very few number of graphene layers are present in the GNS (Fig. 8b).
 | ||
| Fig. 7 Cross section view of graphene nanosheets: (a) PtGNS, which are highly branched and vertically grown and (b) CuGNS electrode, aligned vertically and straight forward. | ||
Various characterisations were carried out on the GNS samples. The XRD spectrum of the GNS sample is given in Fig. 8a. The peak observed at 2θ of ∼21° is assigned as the graphitic peak. The HR-TEM images of graphene flakes are given in Fig. 8b. Samples for the TEM study were prepared by scratching the GNS film deposited over Cu foil and dispersing it in ethanol with ultrasonication for 30 minutes. Thick graphite films and thin graphene flakes were dispersed well, wherein a few-layered light and airy graphene rise to the top. These few-layered GNS were collected, dispersed in ethanol and ultrasonicated for more than 40 min to produce even less few-layered graphene sheets. More than 15 layers of graphene were observed in the TEM image.
Raman spectra of the GNS film on Cu substrate at the initial stage and after the completion of growth are shown in Fig. 8c. The spectra clearly show the graphitization of graphene from the amorphous carbon. From the spectra it is observed that amorphous carbon is present at the initial time of synthesis and later it becomes graphitized. Raman spectra show that the G mode (due to bond stretching between the pairs of sp2 carbon atoms) and D mode (due to breathing modes of sp2 carbon atoms in rings) lie around 1580 and 1360 cm−1, respectively. The D band appears when a finite wave vector mode becomes Raman active in the presence of in plane disorder such as vacancies, stacking faults or finite basal plane dimensions that break translational invariance.41,42 The important feature appearing at approximately 2700 cm−1 is usually called the 2D band in the studies related to graphite and is found in all the materials containing sp2 carbon. The 2D peak of graphene sheet emerges in the GNS spectra when compared to the amorphous carbon. The 2D peak is used to confirm the presence of graphene and this second order Raman spectrum is due to a double resonance process involving two phonons of opposite wave vector.43 Second order peaks include both overtones and combinations of first order peaks44 and are seen at 2700 (2D) and 2930 cm−1 (D + G). There is a blue shift of 13 cm−1 in the G band position when compared to bulk graphite (1581 cm−1) and this shift is attributed to the formation of bulk graphite crystal to graphene sheet.45 The inset (Fig. 8c) shows the Raman spectra from different GNS samples grown on different substrates, such as Pt, Ni, Si, SiO2 and a-SiC, after the completion of growth. All the spectra show clear 2D peaks, which confirm the growth of graphene on all the substrates.
The high resolution 3D AFM image of GNS is shown in Fig. 8d. The morphology of the GNS surface from the AFM image appears to be same as shown in the SEM images of GNS. The line scan of the AFM image for the GNS surface gives the details regarding the thickness of the vertically oriented graphene walls, as shown in Fig. 8e. From the AFM line scan, the thickness of each nanowall tip was observed to be about 15 nm, consisting of more than 20 layers of graphene. Carbon-based nanomaterials, such as carbon nanotubes and graphene, are excellent candidates for superhydrophobic surfaces because of their intrinsically high surface area and nonpolar carbon structure.46 These nanostructured surfaces possess ultrahigh adhesion such as the rose petal effect. Many mechanisms involving the rose petal effect have been revealed such as morphological factors, composite micro-nanostructure, and chemical defects of hydrophobic surfaces.47–49 To observe the hydrophobic properties of the graphene nanosheet matrix, water contact angle (CA) measurements were performed by placing droplets of distilled water on the surface of GNS samples. It was observed that the GNS surface showed strong hydrophobic properties. As shown in Fig. 8f, the optical image of a 2 μl water droplet exhibited a CA of 136°. This strong hydrophobicity was attributed to the surface roughness induced by the morphology of GNS.
4. Conclusion
Highly porous, vertically oriented graphene nanosheets were synthesised using microwave plasma CVD. These graphene nanostructures were shown to have strong hydrophobicity. Nanosheets formed on the surface of the substrate appeared to be parallel to the substrate. Each vertically grown nanowall exhibited more than ∼15 layers of graphene stacked together. The AFM measurements of the surface of GNS showed that the wall thickness was approximately 15 nm. A considerably faster growth rate of GNSs was observed for ECR plasma technique. SEM and HR-TEM images reveal the formation of carbon nanosheet and Raman spectra show the defective nature of the film. This plasma CVD synthesis technique for GNS is very much suitable for energy storage, in particular for the anodes for Li thin film batteries.References
- E. Stratakis, S. Savva, D. Konios, C. Petridi and E. Kymakis, Nanoscale, 2014, 6, 6925–6931 RSC.
- G. Kakavelakis, E. Strataki, D. Konios and E. Kymakis, Chem. Mater., 2014, 26, 5988–5993 CrossRef CAS.
- D. Konios, C. Petridis, G. Kakavelakis, M. Sygletou, K. Savva, E. Stratakis and E. Kymakis, Adv. Funct. Mater., 2015, 25, 2213–2221 CrossRef CAS PubMed.
- N. Balis, D. Konios, E. Stratakis and E. Kymakis, ChemNanoMat, 2015, 1, 346–352 CrossRef PubMed.
- G. Viskadouros, D. Konios, E. Kymakis and E. Stratakis, Appl. Phys. Lett., 2014, 105, 203104 CrossRef PubMed.
- J. Hassoun, F. Bonaccorso and B. Scrosati, et al., Nano Lett., 2014, 14, 4901–4906 CrossRef CAS PubMed.
- F. Schwierz, Nanotechnology, 2010, 5, 487–496 CAS.
- C. Chung, Y. K. Kim, D. Shin and D. H. Min, et al., Acc. Chem. Res., 2013, 46, 2211–2224 CrossRef CAS PubMed.
- V. Tozzini and V. Pellegrini, Phys. Chem. Chem. Phys., 2013, 15, 80–89 RSC.
- D. S. Mao, X. Wang, X. H. Liu, Q. Li and J. F. Xu, Diamond Relat. Mater., 2000, 9, 1876–1880 CrossRef CAS.
- C. Berger, Z. Song, X. Li, X. Wu, N. Brown and C. Naud, et al., Science, 2006, 312, 1191–1196 CrossRef CAS PubMed.
- L. Zhi and K. Mullen, Mater. Chem., 2008, 18, 1472–1484 RSC.
- A. Reina, X. Jia, J. Ho, D. Nezich, H. Son and V. Bulovic, et al., Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed.
- S. J. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS PubMed.
- S. Bae, H. Kim and S. Iijima, et al., Nat. Nanotechnol., 2010, 5, 574–578 CrossRef CAS PubMed.
- M. Yi and Z. Shen, J. Mater. Chem. A, 2015, 3, 11700–11715 CAS.
- W. S. Hummers and R. E. Fffeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. Hernandez, V. Nicolosi and J. N. Coleman, et al., Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed.
- K. V. Emtse, A. Bostwick and T. Seyller, et al., Nat. Mater., 2009, 8, 203–207 CrossRef PubMed.
- S. Gilje, S. Han, K. L. Wang and R. B. Kaner, Nano Lett., 2007, 7, 3394–3398 CrossRef CAS PubMed.
- R. Thomas and G. M. Rao, Electrochim. Acta, 2014, 125, 380–385 CrossRef CAS PubMed.
- R. Thomas, K. Y. Rao and G. M. Rao, Electrochim. Acta, 2013, 108, 458–464 CrossRef CAS PubMed.
- B. Zheng, Y. Yang, J. Chen, K. Yu, J. Yan and K. Cen, Nanoscale, 2013, 5, 5180–5204 RSC.
- Y. Y. Wang, S. Gupta and R. J. Nemanich, Appl. Phys. Lett., 2004, 85, 2601–2603 CrossRef CAS PubMed.
- Y. Wu, P. Qiao, T. Chong and Z. Shen, Adv. Mater., 2002, 14, 64–67 CrossRef CAS.
- S. Virendra, J. Daeha, Z. Lei, D. Soumen, I. K. Saifal and S. Sudipta, Prog. Mater. Sci., 2011, 56, 1178–1271 CrossRef PubMed.
- R. Vitchev, A. Malesevic, R. H. Petrov, R. Kemps, M. Mertens and A. vanhulsel, et al., Nanotechnology, 2010, 21, 095602 CrossRef PubMed.
- K. V. Deenamma and G. M. Rao, Rev. Sci. Instrum., 2000, 71, 467–471 CrossRef PubMed.
- K. Yu, Z. Bo, G. Lu, S. Mao, S. Cui, Y. Zhu, X. Chen, R. S. Ruoff and J. Chen, Nanoscale Res. Lett., 2011, 6, 202 CrossRef PubMed.
- K. Yu, P. Wang, G. Lu, K. H. Chen, Z. Bo and J. Chen, J. Phys. Chem. Lett., 2011, 2, 537–542 CrossRef CAS.
- Z. Bo, K. Yu, G. Lu, S. Cui, S. Mao and J. Chen, Energy Environ. Sci., 2011, 49, 1849–1858 CAS.
- Y. Wu, P. Qiao, T. Chong and Z. Shen, Adv. Mater., 2002, 14, 64–67 CrossRef CAS.
- Y. Wu, Nano Lett., 2002, 2, 355–359 CrossRef CAS.
- S. H. Tsai, C. W. Chao, C. L. Lee and H. C. Shih, Appl. Phys. Lett., 1999, 74, 3462 CrossRef CAS PubMed.
- C. Bower, W. Zhu, S. Jin and O. Zhou, Appl. Phys. Lett., 2000, 77, 830 CrossRef CAS PubMed.
- Z. Wang, M. Shoji and H. Ogata, Appl. Surf. Sci., 2011, 257, 9082–9085 CrossRef CAS PubMed.
- A. Ural, Y. M. Li and H. J. Dai, Appl. Phys. Lett., 2002, 81, 3464–3466 CrossRef CAS PubMed.
- Y. Wu and B. Yang, Nano Lett., 2002, 2, 355–359 CrossRef CAS.
- S. Shimabukuro, Y. Hatakeyama, M. Takeuchi, T. Itoh and S. Nonomura, Thin Solid Films, 2008, 516, 710–713 CrossRef CAS PubMed.
- C. L. Cheng, C. T. Chia, C. C. Chiu and I. N. Lin, Diamond Relat. Mater., 2002, 11, 262–267 CrossRef CAS.
- Z. H. Ni, H. M. Fan, Y. P. Feng, Z. X. Shen, B. J. Yang and Y. H. Wu, J. Chem. Phys., 2006, 124, 204703 CrossRef CAS PubMed.
- R. Saito, A. Jorio, A. G. S. Filho, G. Dresselhaus, M. S. Dresselhaus and M. A. Pimenta, Phys. Rev. Lett., 2001, 88, 027401 CrossRef.
- M. S. Dresselhaus, G. Dresselhaus and M. Hofmann, Philos. Trans. R. Soc., A, 2008, 366, 231–236 CrossRef CAS PubMed.
- R. J. Nemanich and S. A. Solin, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 20, 392–401 CrossRef CAS.
- M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. A. Cancado, A. Jorio and R. Sato, Phys. Chem. Chem. Phys., 2007, 9, 1276–1291 RSC.
- Y. Lin, G. J. Ehlert, C. Bukowsky and H. A. Sodano, ACS Appl. Mater. Interfaces, 2011, 3, 2200–2203 CAS.
- B. Bhushan and E. K. Her, Langmuir, 2010, 26, 8207–8217 CrossRef CAS PubMed.
- M. J. Liu and L. Jiang, Adv. Funct. Mater., 2010, 20, 3753–3764 CrossRef CAS PubMed.
- F. M. Chang, S. J. Hong, Y. J. Sheng and H. K. Tsao, Appl. Phys. Lett., 2009, 95, 064102 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |






