Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two step and one step preparation of porous nanocomposite cellulose membranes doped with TiO2†
Alexandra
Wittmar
*ab,
Dimitri
Vorat
a and
Mathias
Ulbricht
*ab
aLehrstuhl für Technische Chemie II, Universität Duisburg-Essen, 45141 Essen, Germany. E-mail: alexandra.wittmar@uni-due.de; mathias.ulbricht@uni-due.de; Fax: +49 201 183 3147
bCENIDE – Center for Nanointegration Duisburg-Essen, NETZ – NanoEnergieTechnikZentrum, 47057 Duisburg, Germany
First published on 9th October 2015
Abstract
Cellulose–TiO2 nanocomposites have been successfully prepared by non-solvent induced phase separation from cellulose acetate solutions in classical organic solvents followed by deacetylation (“regeneration”). The cellulose deacetylation has been performed either sequentially, i.e. after the completion of the phase separation process, or simultaneously, i.e. during the phase separation process. Commercially available titania nanoparticles from gas phase synthesis processes have been used and processed as a dispersion in the respective polymer solutions. The resulting nanocomposites have been characterized by means of scanning electron microscopy (SEM) and Fourier transform infrared (FT-IR) spectroscopy. Special attention has been given to the complex relation between the conditions of the deacetylation process, the structure of the resulting TiO2 doped cellulose membranes and their corresponding catalytic activities. Two catalytic activity tests, based on the photocatalytic degradation of model organic dyes under UV irradiation, have been used for the functional characterization of the TiO2 doped nanocomposites. The performed experiments demonstrated the successful photocatalyst immobilization in porous cellulose acetate together with good catalytic activity of this nanocomposite intermediate. By simply varying the conditions of the cellulose deacetylation, nanocomposite cellulose membranes with different structures and properties have been obtained. However after the regeneration of cellulose a partial decrease of the catalytic activity was observed.
1. Introduction
Cellulose is one of the most widely available natural polymers and together with its derivatives has already many established areas of applicability such as in paper manufacturing,1 artificial fibers and textiles,2,3 membranes for water purification4,5 and/or biomedical analyses and therapies.6,7 Cellulose-based materials with new functionalities have continuously attracted the interest of chemistry researchers, following the current trends to utilize renewable and environmentally friendly, abundantly available and biodegradable materials in all fields of application. Doping cellulose and derivatives with inorganic nanoparticles leads to the formation of inorganic–organic hybrid nanocomposites which by the synergistic combination of the components' characteristics can exhibit additional functionalities as well as superior thermal, mechanical, electrical and optical properties. This way the area of applicability of the cellulose based materials can be widely expanded. For example, doping cellulose with silica or montmorillonite, nanocomposites with improved thermal and mechanical stability have been obtained,8,9 and by doping cellulose with SnO2 materials for chemical, humidity and biomarker sensing have been produced.10 Ferromagnetic nanoparticles like MnFe2O4 confer cellulose water repellent and superparamagnetic properties as well as magnetic responsivity.11 ZnO and Ag+ confer cellulose biocompatibility and antibacterial activity12,13 and TiO2 doping provides antibacterial14 and catalytic activity.15One of the materials with a huge role in nanoscience and nanotechnology is TiO2. Among its main areas of applications worth mentioning are catalysis,16 solar cells,17 pigments,18 corrosion protection19 or optical coatings.20 TiO2 is one of the mostly used photocatalysts for the decomposition of organic and inorganic contaminants like Cr(VI) in wastewater due to its superior properties: high chemical stability, low toxicity and low cost.21,22 Widespread are three polymorphic forms of TiO2: anatase, rutile and brookite, and some commercial powders usually consist of mixtures of anatase and rutile. The bandgap of rutile is ∼3.0 eV and that of anatase is ∼3.2 eV.23 Generally it is considered that in pure form anatase exhibits superior photocatalytic activity.24 In depth studies have demonstrated that phase mixtures of different TiO2 polymorphs have increased photocatalytic activity when compared with pure phases.25
A wide range of cellulose-based nanocomposites doped with TiO2 have been prepared by different processes and have found different applications like: nanohybrid membranes with improved porosity, stability and separation performance;26 self-cleaning materials and purification filters with predefined hydrophilicity;27 nanocomposites with high bactericidal activity28,29 and nanocomposites with photocatalytic activity.30–32 In one of the most recent applications, the TiO2 doped cellulose materials were used for water purification by photocatalytic reactions.33
Cellulose is insoluble in water and in most of the conventional organic solvents and it decomposes before reaching the melting temperature; therefore processing of cellulose in unmodified form is rather difficult. The main reason for cellulose insolubility is connected with its high crystalline content which is strongly connected to the dense chain packing due to intra- and intermolecular hydrogen bonding. The traditional methods for the preparation of “regenerated” cellulose materials include cellulose dissolution in aqueous xanthate solutions, or solutions of cellulose in lithium chloride–dimethylacetamide mixtures, sodium hydroxide–thiourea aqueous solutions or tetraalkylammonium chloride solutions in organic solvents. More recent studies were dedicated to the dissolution of cellulose in a new class of non-derivatizing solvents–room temperature ionic liquids.34
Some of the above mentioned processes are hazardous to the environment; some involve relatively expensive solvents or imply difficult processing due to high viscosity of the cellulose solutions. In contrast to cellulose, cellulose acetate has less hydrogen bonds and a lower crystallinity; this makes it easily soluble in a wide range of organic solvents like acetone, dimethylformamide (DMF), dimethylsulfoxide (DMSO) etc.
One of the most widely spread methods for porous films and membranes preparation is the phase separation from a polymer solution either by thermally induced, vapor induced or by non-solvent immersion precipitation processes. In non-solvent induced phase separation, a polymer solution which may contain other additives is cast on a flat substrate as a thin film. Subsequently the film is immersed in a coagulation bath which contains the non-solvent, may also contain other additives and is highly miscible with the polymer solvent. The final pore morphology of the formed membrane depends on the exchange between the solvent and the non-solvent, influenced by the composition of the polymer solution and the coagulation bath, by the polymer concentration in solution, by the solvent and non-solvent volatility, etc.35,36 Very recently a research group from Malaysia has reported the successful preparation by non-solvent induced phase separation of cellulose membranes loaded with N-doped TiO2 from a cellulose solution in an ionic liquid. The obtained membranes very efficiently degraded organic dye methylene blue under UV and visible irradiation.37,38 The main downside of the cellulose membrane formation from solutions in ionic liquids is given by the high viscosity of the polymer solution which makes the control of pore formation difficult.39 In one of our previous works we reported on two alternative routes which facilitate the preparation of catalytically active cellulose membranes using commercial TiO2 nanoparticles as active functional material: one starting with cellulose solutions in ionic liquids and the second starting with cellulose acetate solutions in conventional solvents.39 In the present paper we analyze in depth the possibilities to prepare TiO2 doped porous cellulose membranes, starting with TiO2 doped cellulose acetate solutions in conventional solvents which are transformed in porous nanocomposites by phase separation process. The cellulose is simply regenerated by alkaline treatment of the cellulose acetate intermediate, either directly during the phase separation process or in a subsequent separate step. The proposed processes combine the advantage of cellulose acetates' facile solubilisation in organic solvents with the use of well dispersed cheap commercially available catalytically active TiO2 nanoparticles and the facile design of porous membrane structure by the choice of phase separation conditions. The intermediate cellulose acetate nanocomposite membranes possess catalytic activity and may find direct applications in water purification processes or self-cleaning surfaces. A partial loss of catalytic activity after the deacetylation process has been observed; therefore future studies concerning the optimal deacetylation conditions or the post-synthesis reactivation of the catalyst are necessary.
2. Materials and methods
2.1. Materials
Cellulose acetate (CA) with a molecular weight Mn ∼ 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and an acetyl content of 39.8 wt%, as given by the provider, was supplied by Sigma-Aldrich. Cellulose acetate solutions were prepared in acetone (p.a.) and dimethylformamide (DMF; p.a.), both supplied by VWR International. Titan dioxide nanoparticles TiO2 Aeroxide P90 were kindly offered by Evonik Industries, TiO2 Hombikat 8630 and TiO2 Hombikat 8602 were kindly offered by Sachtleben Chemie GmbH. The dyes used for the catalytic activity tests were methylene blue (hydrate; ≥95%) from Fluka and rhodamine B (96%) from Alfa Aesar. During the cellulose regeneration process KOH (p.a.), HCl 0.1 M (standard solution) and ethanol (96%, Ph. Eur.) from VWR International were used.
000 g mol−1 and an acetyl content of 39.8 wt%, as given by the provider, was supplied by Sigma-Aldrich. Cellulose acetate solutions were prepared in acetone (p.a.) and dimethylformamide (DMF; p.a.), both supplied by VWR International. Titan dioxide nanoparticles TiO2 Aeroxide P90 were kindly offered by Evonik Industries, TiO2 Hombikat 8630 and TiO2 Hombikat 8602 were kindly offered by Sachtleben Chemie GmbH. The dyes used for the catalytic activity tests were methylene blue (hydrate; ≥95%) from Fluka and rhodamine B (96%) from Alfa Aesar. During the cellulose regeneration process KOH (p.a.), HCl 0.1 M (standard solution) and ethanol (96%, Ph. Eur.) from VWR International were used.
2.2. Preparation of cellulose based membranes
The TiO2 dispersions in acetone and DMF were prepared at the desired concentrations (10, 15 and 20 wt% with respect to the polymer) by treatment with a Bandelin Sonoplus Generator GM 2200 ultrasound homogenisator. The solvents and the corresponding amount of nanoparticle powder were introduced in a glass vial and then homogenized for 2 × 5 min with amplitude of 30%. Between the sonication intervals a pause of 5 min was necessary in order to avoid the overheating of the dispersion.For the preparation of the polymer solutions (with or without nanoparticles) the solvent or nanoparticle dispersions were mixed with the desired amount of polymer (16 wt% with respect to the solvent) and homogenized in a mortar, and then the samples were stirred at room temperature until complete polymer dissolution.
The polymer films were casted on glass substrates with the help of a motorized film applicator (model AB3400 from TQC), using a casting knife with gap width of 300 μm and a speed of 20 mm min−1. The glass supported polymer films were immediately immersed (average residence time after casting ca. 30 s) in the coagulation bath consisting of distilled water at room temperature and were left there for ca. 24 h in order to allow completion of phase separation. When the membrane deacetylation was performed simultaneously with the membrane precipitation, the polymer film was immersed in a KOH solution in water or in water/EtOH mixture. When the deacetylation was performed post preparation, the cellulose acetate wet membrane was transferred in a KOH water bath of known concentration. Different KOH concentrations in the coagulation bath (0.05 N, 0.1 N, 0.2 N, 0.25 N and 0.5 N) and different regeneration time intervals (between 1 h and 24 h) have been studied. Subsequently, the membranes were washed with fresh distilled water and dried in air at room temperature.
2.3. Characterization methods
The agglomerate size in dispersion, dDLS, was determined by dynamic light scattering (DLS) method using a Particle Metrix Stabisizer heterodyne backscattering equipment at a laser wavelength of 500 nm and a laser power of 5 mW. Each sample was measured three times over a period of 60 s for each run and the result is the average of the three measurements. The DLS data are presented as distribution by number, but the distribution by intensity was also recorded for a better interpretation of the results.Scanning electron micrographs of the porous membranes at different magnifications were taken with a FEI ESEM Quanta 400 FEG instrument. For the cross section measurements the samples were broken in liquid nitrogen. The samples were sputtered with Au/Pd (80/20) at 0.1 mbar and 30 mA for 30 s until a layer of 2–3 nm was obtained.
The results of the deacetylation process were evaluated with help of FT-IR ATR spectroscopy using a Varian 3100 Excalibur series spectrometer with an angle of incidence of 45° and a diamond crystal. The 600–4000 cm−1 spectral range was measured with at an average of 32 scans per sample and with a resolution of 4 cm−1.
The catalytic activity of the nanoparticle doped cellulose-based nanocomposites has been evaluated in the photodegradation of organic dyes like methylene blue or rhodamine B in two tests:
3. Results and discussion
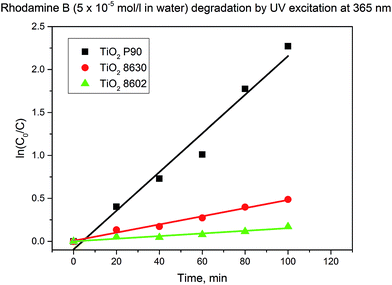
The TiO2 P90 nanoparticles are preponderantly constituted from anatase phase, i.e. ca. 97% (primary particle size for anatase is 10–14 nm) and only ca. 3% rutile (primary particle size for rutile is 12–17 nm) content. These particle size distributions calculated from the XRD patterns are in good agreement with the observations in transmission electron micrographs.32 According to producer specifications, the TiO2 Hombikat 8630 is constituted from a fine particulate anatase with 5% WO3 and the TiO2 Hombikat 8602 is a fine particulate titanium oxide hydrate (primary particles size 5–10 nm) with anatase structure and very large specific surface area >250 m2 g−1.The agglomeration of the TiO2 nanoparticles in the solvents of interest was analyzed by dynamic light scattering DLS (see ESI, Fig. SI-1†). The three types of TiO2 disperse differently in DMF, the average agglomerate size is increasing in the following order: TiO2 P90 < TiO2 Hombikat 8630 < TiO2 Hombikat 8602. The average agglomerate size for TiO2 P90 was ca. 100 nm in DMF and 200 nm in acetone.
As consequence of different compositions and properties, the three TiO2 materials interact differently with the organic dyes under UV excitation at 365 nm. For example for the decomposition of rhodamine B, TiO2 P90 is the most active material, followed by the Hombikat 8630 and finally by the Hombikat 8602 (Fig. 1). Photodegradation by UV excitation at 365 nm of rhodamine B in the absence of the catalyst was not observed for irradiation times up to 2 h (see ESI, Fig. SI-2†).
With all three types of titanium dioxide nanopowders cellulose acetate-based membranes with a composition CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10
TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 have been prepared by phase separation from polymer solutions in DMF. Membranes with similar pore structure have been obtain in all cases; however differences in the agglomeration degree of the TiO2 dopant in the porous membranes have been observed (see ESI, Fig. SI-3†). As expected, the membranes have accordingly different catalytic properties (see ESI, Fig. SI-4†); however it is very interesting to observe that by embedment in the cellulose acetate matrix, the TiO2 Hombikat 8602 loses its catalytic activity to the smallest extent (see ESI, Fig. SI-5†).
2 have been prepared by phase separation from polymer solutions in DMF. Membranes with similar pore structure have been obtain in all cases; however differences in the agglomeration degree of the TiO2 dopant in the porous membranes have been observed (see ESI, Fig. SI-3†). As expected, the membranes have accordingly different catalytic properties (see ESI, Fig. SI-4†); however it is very interesting to observe that by embedment in the cellulose acetate matrix, the TiO2 Hombikat 8602 loses its catalytic activity to the smallest extent (see ESI, Fig. SI-5†).
Because the catalytic results are more promising for the membranes obtained using TiO2 90, the further investigations have been performed using this catalyst.
By dissolution of CA in the TiO2 P90 dispersions in DMF and acetone, casting solutions with different viscosities have been obtained: 1.100 cP in DMF and 500 cP in acetone. The rate of the demixing process affects the morphology of the formed membrane. This can be also observed in the different morphologies of the CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 membranes formed from acetone and DMF polymer solutions and using water as non-solvent (Fig. 2). When DMF is used as solvent, instantaneous demixing takes place leading to the formation of a membrane with macrovoids.32 When acetone solutions are used, due to high volatility of acetone and fast water diffusivity, delayed demixing occurs and membranes with sponge-like structure are formed.32
TiO2 membranes formed from acetone and DMF polymer solutions and using water as non-solvent (Fig. 2). When DMF is used as solvent, instantaneous demixing takes place leading to the formation of a membrane with macrovoids.32 When acetone solutions are used, due to high volatility of acetone and fast water diffusivity, delayed demixing occurs and membranes with sponge-like structure are formed.32
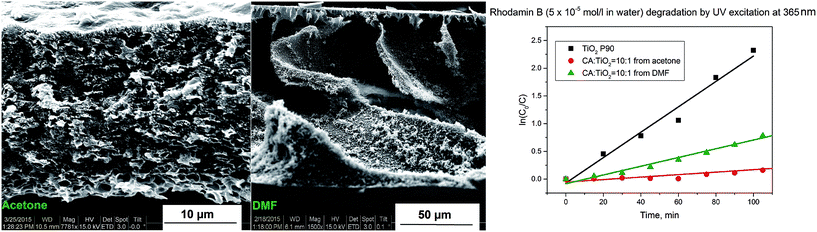 | ||
Fig. 2 CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 (ratio 10 TiO2 (ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) membranes prepared from acetone and DMF solutions: cross section structures and catalytic activity (note the different magnifications in the two SEM micrographs). 1) membranes prepared from acetone and DMF solutions: cross section structures and catalytic activity (note the different magnifications in the two SEM micrographs). | ||
As observed from the Fig. 2, both membranes exhibit catalytic activity, which, however, is lower when compared with the same mass of pure catalyst. The main reason is the loss of active surface of the catalyst by embedment in the polymer matrix. The catalytic activity of the membrane prepared from DMF solution is three times higher (Table 1) as compared to the equivalent membrane prepared from acetone solution. This reduced catalytic activity of the membrane prepared from acetone based solution may be connected with higher agglomeration of the TiO2 in acetone (see ESI, Fig. SI-1†), but also with the lower porosity of the membrane surfaces (see ESI, Fig. SI-6†). Additionally, the membrane prepared from acetone solution is slightly less hydrophilic than the equivalent one prepared from DMF solution (see ESI, Fig. SI-7†).
| Sample | Deacetylation | k [min−1] |
|---|---|---|
| TiO2 P90 | — | 0.0225 ± 0.0018 |
| TiO2 8630 | — | 0.0048 ± 0.0003 |
| TiO2 8602 | — | 0.0015 ± 0.0002 |
| TiO2 P90 | 0.2 N KOH in H2O | 0.0020 ± 0.0005 |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (acetone) 1 (acetone) |
— | 0.0022 ± 0.0005 |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMF) 1 (DMF) |
— | 0.0079 ± 0.0004 |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (DMF) 1.5 (DMF) |
— | 0.0168 ± 0.0004 |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (DMF) 2 (DMF) |
— | 0.0140 ± 0.0002 |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMF) 1 (DMF) |
0.5 N KOH in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH |
Near detection limit |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMF) 1 (DMF) |
Direct phase separation in 0.25 N KOH in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH |
Near detection limit |
CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 = 10 TiO2 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMF) 1 (DMF) |
Direct phase separation in 0.2 N KOH in H2O | Near detection limit |
Because the catalytic results are more promising for the membranes obtained by phase separation from polymer solutions in DMF, the further investigations have been performed on this type of membranes.
The influence of the TiO2 content on the phase separation process and on the resulting membrane characteristics was studied in detail. No significant change in the outcome of the phase separation process was observed with the increase of the nanocatalyst content in the membrane; however a slightly larger density of catalyst agglomerates within the polymer and a slight increase in the agglomerate size was observed at higher concentrations of TiO2 (Fig. 3). This observation is in good agreement with the results of the DLS investigations performed on dispersions with different contents of TiO2 in DMF where a pronounced increase in average agglomerate size from ca. 100 nm at 1% to a main size in the range of 300 nm and a very broad distribution had been observed (see ESI, Fig. SI-8†). The general tendency is that the agglomerate sizes are increasing with the increase of the nanopowder concentration. Additionally, with the increase of the nanoparticle fraction in the nanocomposite a slight decrease in the hydrophilicity of the membrane was observed (see ESI, Fig. SI-9†).
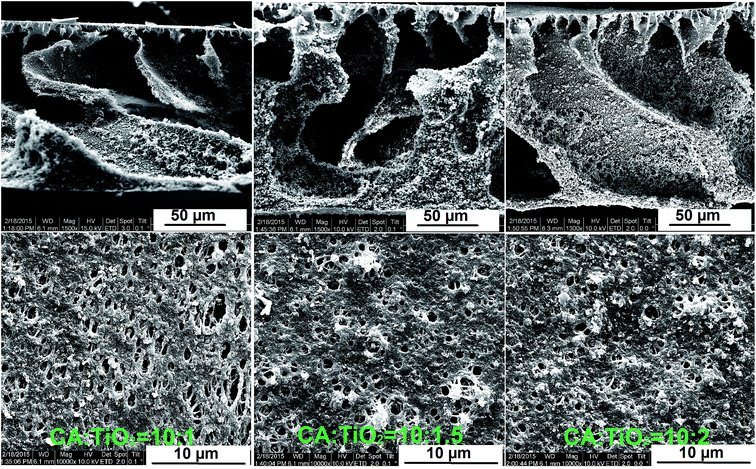 | ||
Fig. 3 Scanning electron microscopy images (cross section and glass surface) of CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 membranes with different TiO2 concentrations. TiO2 membranes with different TiO2 concentrations. | ||
All membranes with different TiO2 contents have shown good catalytic activity. At the increase of the TiO2 concentration from 10 wt% to 15 wt% the catalytic activity doubled. At the further increase of the TiO2 concentration to 20 wt% a slight decrease of the catalytic activity in comparison to the membrane with 15 wt% TiO2 was observed (Fig. 4 and Table 1).
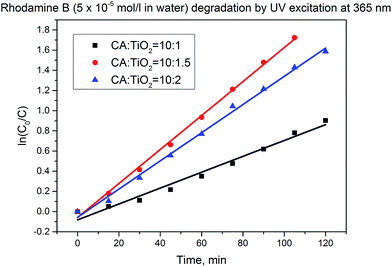 | ||
Fig. 4 Rhodamine B degradation with CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 membranes (dried nanocomposite) having different TiO2 content (same amount of composite). TiO2 membranes (dried nanocomposite) having different TiO2 content (same amount of composite). | ||
When evaluating the catalytic activity of the CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 porous nanocomposites, the following aspects should be taken into consideration: by increasing the amount of TiO2 in the material, the amount of catalytically active species in the composite increases; therefore the resulted membrane is more catalytically active. However, too high TiO2 concentration will lead to strong agglomeration of the nanopowder in the composite, having as consequence the reduction of the available catalytically active surface. This effect is much more pronounced than the possible negative effect generated by the slight decrease of hydrophilicity. Therefore, for each type of membrane, studies are necessary in order to identify the optimal amount of catalytically active material which should be embedded in the membrane. Here, the loading of 15% TiO2 relative to cellulose acetate seemed to be close to the optimum.
TiO2 porous nanocomposites, the following aspects should be taken into consideration: by increasing the amount of TiO2 in the material, the amount of catalytically active species in the composite increases; therefore the resulted membrane is more catalytically active. However, too high TiO2 concentration will lead to strong agglomeration of the nanopowder in the composite, having as consequence the reduction of the available catalytically active surface. This effect is much more pronounced than the possible negative effect generated by the slight decrease of hydrophilicity. Therefore, for each type of membrane, studies are necessary in order to identify the optimal amount of catalytically active material which should be embedded in the membrane. Here, the loading of 15% TiO2 relative to cellulose acetate seemed to be close to the optimum.
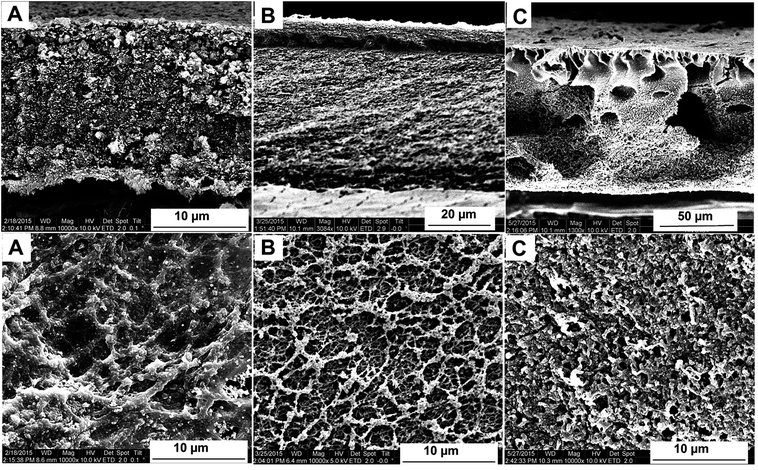
Different TiO2 doped cellulose membranes have been prepared by deacetylation of the corresponding cellulose acetate membranes, either by deacetylation post precipitation (Fig. 5A), or by simultaneous deacetylation and precipitation (Fig. 5B and C). Depending on the chosen method of deacetylation, membranes with different porous structure have been obtained (Fig. 5). The success of the deacetylation process was assessed by FT-IR spectroscopy. In all cases it was observed that the peaks at ∼1740 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch vibration and ∼1220 cm−1 corresponding to the C–O vibration, both for the ester groups, are disappearing from the composite spectrum (see ESI, Fig. SI-10†). In the two step process, the changes in the original membrane structure after the cellulose regeneration by alkaline treatment have been attributed by Park and collaborators to the rearrangement of the polymer chains during the deacetylation and to a change of the solid state structure of CA toward crystalline phases of cellulose.40 In the one step process, the polymer precipitation and its structural change and organization take place simultaneously.
O stretch vibration and ∼1220 cm−1 corresponding to the C–O vibration, both for the ester groups, are disappearing from the composite spectrum (see ESI, Fig. SI-10†). In the two step process, the changes in the original membrane structure after the cellulose regeneration by alkaline treatment have been attributed by Park and collaborators to the rearrangement of the polymer chains during the deacetylation and to a change of the solid state structure of CA toward crystalline phases of cellulose.40 In the one step process, the polymer precipitation and its structural change and organization take place simultaneously.
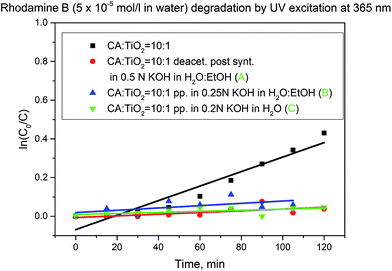
Regardless of the method of deacetylation applied, all the formed TiO2 doped cellulose membranes had considerably lower catalytic activity when compared with the cellulose acetate based intermediates (Fig. 6 and Table 1).
Literature mentions that the reaction of TiO2 with KOH leads to poisoning of Lewis acid sites and surface hydroxyl groups.41 In order to prove this hypothesis, pure TiO2 P90 was treated with a 0.2 N KOH solution, then separated by centrifugation, thoroughly washed with distilled water and finally dried. The catalytic activity of the KOH treated TiO2 powder was tested in analogous manner as for the native material. As in the case of the deacetylated membranes a decrease of the catalytic activity of the material was observed, in line with the poisoning of the catalyst (Fig. SI-11†). Partially, this decrease of catalytic activity may also be connected with the aggregation of the TiO2 nanoparticles after the alkaline treatment.
As a next step, an optimization of the deacetylation process was performed with the aim to establish conditions where the exposure of the nanocomposite to the KOH solution is minimized or to find a viable route which will allow catalyst “reactivation” after the completion of the deacetylation step (e.g. treatment of the membrane with a diluted acid).
Firstly, it was investigated which is the minimal exposure time to KOH solutions of different concentrations necessary for a complete deacetylation of the cellulose acetate intermediate. Therefore cellulose acetate membranes were exposed to the desired alkaline solution for fixed amounts of time, then thoroughly washed and finally dried. The required time for complete deacetylation was ca. 260 min at the immersion in 0.05 N aqueous KOH and ca. 90 min at the immersion in 0.1 N aqueous KOH solutions (Fig. 7; see also ESI, Fig. SI-12†).
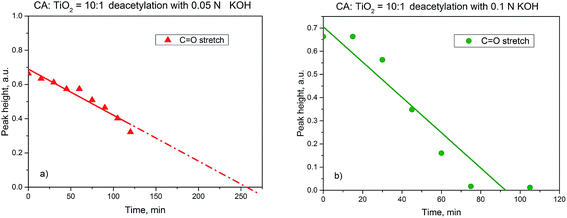 | ||
Fig. 7 Height of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch peak in the membranes FT-IR spectrum vs. time for deacetylation in 0.05 N aqueous KOH (a) and 0.1 N aqueous KOH (b) solutions. O stretch peak in the membranes FT-IR spectrum vs. time for deacetylation in 0.05 N aqueous KOH (a) and 0.1 N aqueous KOH (b) solutions. | ||
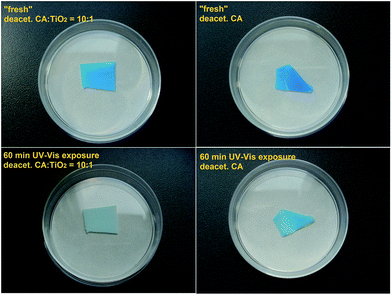
Regardless of the concentration of alkaline solution and deacetylation time, all regenerated cellulose membranes have shown much lower catalytic activity than their cellulose acetate precursor. The “Test 1” has, however, clearly shown that even though the membranes are considerably less active after deacetylation, the photocatalytic activity is not completely lost. A slow decomposition of the organic dye adsorbed in the membrane under UV-Vis exposure (sunlight) has been observed, as confirmed by exemplary data shown in Fig. 8 left. In order to prove that it is not a case of dye bleach, the same test was performed on deacetylated cellulose without TiO2. In the absence of the catalyst, decoloration upon UV-Vis exposure (sunlight) was very much less pronounced (Fig. 8 right).
Attempts toward reactivation of activity of cellulose nanocomposites with dilute aqueous HCl had not been successful. Different other post-deacetylation treatments, aiming the reactivation of the TiO2, will be the objective of planned future work.
4. Conclusions
In the present work, the potential offered by the two step and the one step preparation methods of TiO2 doped porous cellulose nanocomposites, starting with nanoparticle dispersions in cellulose acetate solutions in organic solvents, had been analyzed in detail. TiO2 doped porous cellulose acetate nanocomposite membranes have been prepared by phase separation from polymer solutions in organic solvents using water as precipitation medium. The obtained nanocomposite membranes can directly be used as catalytically active materials or can serve in the second step as precursor for the preparation of TiO2 doped porous cellulose membranes. The cellulose regeneration from cellulose acetate based materials was simply realized by alkaline treatment. Three types of catalytically active commercial titanium dioxide nanopowders have been investigated and it was observed an increase of the photocatalytic activity for degradation of organic dyes in the following order: Hombikat 8602 < Hombikat 8630 < P90. A similar increase of the catalytic activity was also obtained for the corresponding cellulose acetate based membranes. As expected, by simply changing the cellulose acetate solvent it was possible to change the membrane porous structure. The addition of inorganic nanoparticles did not have a considerable influence of the porous membrane formation during the phase separation process. It was observed that the membrane prepared from DMF solution has considerably better catalytic activity than the corresponding membrane obtained from acetone solution. By increasing the amount of the TiO2 in the CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 nanocomposite an increase of the photocatalytic activity of the membrane was observed until a certain value. Increase of TiO2 content beyond that level lead to lower catalytic activity. Therefore the optimal doping amount for each type of membranes should be determined. Sequential deacetylation after phase separation process leads to a partial collapsing of the pores in the resulted TiO2 doped cellulose-based membranes. Similar structures as the ones obtained for the cellulose acetate intermediates can be also obtained for the cellulose membranes when the deacetylation takes place simultaneously with the phase separation of films of cellulose acetate solutions. By increasing the alkali concentration, the rate of the deacetylation process can be increased. Regardless of the route used for the cellulose regeneration, a decrease of catalytic activity of the resulted TiO2 doped nanocomposite membranes was observed, apparently due to TiO2 poisoning. The huge potential of this approach is given by relatively cheap materials which are available at larger scale (good potential for up-scaling) as well as by the very large number of possibilities to influence the membrane structure formation for different targeted applications due to wide range of solvents, many possible compositions of the coagulation bath and various possible compositions of the deacetylation bath. Studies concerning the optimization of the deacetylation process and about the “reactivation” of the catalyst in the cellulose-based composites will make the objective of future studies. Additionally, the TiO2 doped porous cellulose will be used as precursor for the preparation of catalytically active carbon based nanocomposites.
TiO2 nanocomposite an increase of the photocatalytic activity of the membrane was observed until a certain value. Increase of TiO2 content beyond that level lead to lower catalytic activity. Therefore the optimal doping amount for each type of membranes should be determined. Sequential deacetylation after phase separation process leads to a partial collapsing of the pores in the resulted TiO2 doped cellulose-based membranes. Similar structures as the ones obtained for the cellulose acetate intermediates can be also obtained for the cellulose membranes when the deacetylation takes place simultaneously with the phase separation of films of cellulose acetate solutions. By increasing the alkali concentration, the rate of the deacetylation process can be increased. Regardless of the route used for the cellulose regeneration, a decrease of catalytic activity of the resulted TiO2 doped nanocomposite membranes was observed, apparently due to TiO2 poisoning. The huge potential of this approach is given by relatively cheap materials which are available at larger scale (good potential for up-scaling) as well as by the very large number of possibilities to influence the membrane structure formation for different targeted applications due to wide range of solvents, many possible compositions of the coagulation bath and various possible compositions of the deacetylation bath. Studies concerning the optimization of the deacetylation process and about the “reactivation” of the catalyst in the cellulose-based composites will make the objective of future studies. Additionally, the TiO2 doped porous cellulose will be used as precursor for the preparation of catalytically active carbon based nanocomposites.
Acknowledgements
We gratefully acknowledge the collaboration with Mr Smail Boukercha (SEM characterization) at the University of Duisburg-Essen.References
- J. Langley and D. Holroyd, US. Pat., 4 913 775, 1990.
- R. C. Law, Macromol. Symp., 2004, 208, 255 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358 CrossRef CAS PubMed.
- H. Ma, C. Burger, B. S. Hsiao and B. Chu, J. Mater. Chem., 2011, 21, 7507 RSC.
- G. M. Geise, H. S. Lee, D. J. Miller, B. D. Freeman, J. E. McGrath and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 1685 CrossRef CAS.
- N. Hoenich, BioResources, 2006, 1, 270 Search PubMed.
- Q. Wang, A. Fu, H. Li, J. Liu, P. Guo, X. S. Zhao and L. H. Xia, Carbohydr. Polym., 2014, 111, 393 CrossRef CAS PubMed.
- S. Mahmoudian, M. U. Wahit, A. F. Ismail and A. A. Yussuf, Carbohydr. Polym., 2012, 88, 1251 CrossRef CAS.
- H. Song and L. Zheng, Cellulose, 2013, 20, 1737 CrossRef CAS.
- S. K. Mahadeva and J. Kim, Sens. Actuators, B, 2011, 157, 177 CrossRef CAS.
- D. Fragouli, I. S. Bayer, R. Di Corato, R. Brescia, G. Bertoni, C. Innocenti, D. Gatteschi, T. Pellegrino, R. Cingolani and A. Athanassiou, J. Mater. Chem., 2012, 22, 1662 RSC.
- M. Ul-Islam, W. A. Khattak, M. W. Ullah, S. Khan and J. K. Park, Cellulose, 2014, 21, 433 CrossRef CAS.
- S. M. Li, L. H. Fu, M. G. Ma, J. F. Zhu, R. C. Sun and F. Xu, Biomass Bioenergy, 2012, 47, 516 CrossRef CAS.
- S. M. Li, Y. Y. Dong, M. G. Ma, L. H. Fu, R. C. Sun and F. Xu, Carbohydr. Polym., 2013, 96, 15 CrossRef CAS PubMed.
- J. Zheng, S. Liu, J. Cai and L. Zhang, J. Phys. Chem. C, 2010, 114, 7806 Search PubMed.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269 CrossRef CAS.
- G. K. Mor, O. K. Varghese, M. Paulose, K. Shankar and C. A. Grimes, Sol. Energy Mater. Sol. Cells, 2006, 90, 2011 CrossRef CAS.
- G. Pfaff and P. Reynders, Chem. Rev., 1999, 99, 1963 CrossRef CAS PubMed.
- G. X. Shen, Y. C. Chen and C. J. Lin, Thin Solid Films, 2005, 489, 130 CrossRef CAS.
- C. Euvananont, C. Junin, K. Impor, P. Limthongkul and C. Thanachayanont, Ceram. Interfaces, 2008, 34, 1067 CrossRef CAS.
- Y. C. Zhang, M. Yang, G. Zhang and D. D. Dionysiou, Appl. Catal., B, 2013, 142–143, 249 CrossRef CAS.
- A. Turki, C. Guillard, F. Dappozze, Z. Ksibi, G. Berhault and H. Kochkar, Appl. Catal., B, 2015, 163, 404 CrossRef CAS.
- M. Pelaez, N. T. Nolan, S. C. Pillai, M. K. Seery, P. Falaras, A. G. Kontos, P. S. M. Dunlop, J. W. J. Hamilton, J. A. Byrne, K. O'Shea, M. H. Entezari and D. D. Dionysiou, Appl. Catal., B, 2012, 125, 331 CrossRef CAS.
- T. Luttrell, S. Halpegamage, J. Tao, A. Kramer, E. Sutter and M. Batzill, Sci. Rep., 2014, 4, 4043 Search PubMed.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. A. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Nat. Mater., 2013, 12, 798 CrossRef CAS PubMed.
- R. Abedini, S. M. Mousavi and A. Aminzadeh, Desalination, 2011, 277, 40 CrossRef CAS.
- G. Goncalves, P. A. A. P. Marques, R. J. B. Pinto, T. Trindade and C. P. Neto, Compos. Sci. Technol., 2009, 69, 1051 CrossRef CAS.
- W. A. Daoud, J. H. Xin and Y. H. Zhang, Surf. Sci., 2005, 599, 69 CrossRef CAS.
- S. M. Li, Y. Y. Dong, M. G. Ma, L. H. Fu, R. C. Sun and F. Xu, Carbohydr. Polym., 2013, 96, 15 CrossRef CAS PubMed.
- J. Zeng, S. Liu, J. Cai and L. Zhang, J. Phys. Chem. C, 2010, 114, 7806 CAS.
- T. Zhu, Y. Lin, Y. Luo, X. Hu, W. Lin, P. Yu and C. Huang, Carbohydr. Polym., 2012, 87, 901 CrossRef CAS.
- Y. Luo, J. Xu and J. Huang, CrystEngComm, 2014, 16, 464 RSC.
- S. D. Wang, Q. Ma, H. Liu, K. Wang, L. Z. Ling and K. Q. Zhang, RSC Adv., 2015, 5, 40521 RSC.
- T. Liebert, ACS Symp. Ser., 2010, 1033, 3 CrossRef CAS.
- P. van der Witte, P. J. Dijkstra, J. W. A. van der Berg and J. Feijen, J. Membr. Sci., 1996, 117, 1 CrossRef.
- G. R. Guillen, Y. Pan, M. Li and E. M. V. Hoek, Ind. Eng. Chem. Res., 2011, 50, 3798 CrossRef CAS.
- M. A. Mohamed, W. N. W. Salleh, J. Jaafar, A. F. Ismail, M. A. Mutalib and S. M. Jamil, Carbohydr. Polym., 2015, 133, 429 CrossRef CAS PubMed.
- M. A. Mohamed, W. N. W. Salleh, J. Jaafar, A. F. Ismail, M. A. Mutalib, N. A. A. Sani, S. E. A. M. Asri and C. S. Ong, Chem. Eng. J., 2016, 284, 202 CrossRef CAS.
- A. Wittmar, H. Thierfeld, S. Köcher and M. Ulbricht, RSC Adv., 2015, 5, 35866 RSC.
- W. K. Son, J. H. Youk, T. S. Lee and W. H. Park, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 5 CrossRef CAS.
- B. Imelik, C. Iaccache, G. Coundrier, Y. Ben Taarit and J. C. Vedrine, Catalysis by acids and bases, Elsevier Science Publishers B.V., 1985, p. 22 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Particle size distribution (DLS) of different TiO2 nanoparticles in dimethylformamide; particle size distribution (DLS) of TiO2 P90 in different solvents; particle size distribution (DLS) of TiO2 P90 in different concentrations in dimethylformamide; SEM images of cellulose acetate–TiO2 nanocomposites with different types of TiO2; SEM images of the surfaces of cellulose acetate–TiO2 membranes prepared from acetone and dimethylformamide polymer solutions; water contact angles of cellulose acetate–TiO2 membranes prepared from polymer solutions in acetone and dimethylformamide; FT-IR spectra of different cellulose acetate–TiO2 membranes before, during and after the deacetylation; additional results regarding the catalytic activity of nanocomposites. See DOI: 10.1039/c5ra16337d |
| This journal is © The Royal Society of Chemistry 2015 |