Towards understanding the stability of the N5+-containing salts: the role of counterions†
Yi Yua,
Yu-chuan Lia,
Ji-feng Chena,
Cheng-hui Suna,
Jin-shan Lic,
Gui-juan Fanc,
Si-ping Pang*a and
Ru-bo Zhang*b
aSchool of Materials Science & Engineering, Beijing Institute of Technology, South Street No. 5, Zhongguancun, Haidian District 100081, Beijing, China. E-mail: pangsp@bit.edu.cn; Web: http://pnc.bit.edu.cn/
bSchool of Chemistry, Beijing Institute of Technology, South Street No. 5, Zhongguancun, Haidian District 100081, Beijing, China. E-mail: zhangrubo@bit.edu.cn
cInstitute of Chemical Materials, China Academy of Engineering Physics, P. O. Box 919-327, Mianyang, Sichuan, 621900, China
First published on 1st December 2015
Abstract
The thermal stabilities of the N5+M− species (M = Sb(OH)6, Sb(OH)4F2, AlF6, AlF4, BF4, B(CF3)4, PF6, AsF6 and SbF6) have been studied by means of density functional theory. The present calculations indicate that their thermal stabilities (represented by activation enthalpy, ΔH≠ in kcal mol−1) decrease in the order N5+ (47.2) > N5B(CF3)4 (34.1) ≈ N5SbF6 (31.6) ≈ N5AsF6 (31.5) > N5PF6 (30.5) > N5AlF4 (27.1) ≈ N5BF4 (27.1) > N5AlF6 (8.5). Only N5SbF6 has a small positive reaction enthalpy, ΔrH. The thermal stability of the N5+ salt depends on the amount of electron transfer from the counterion to N5+ in the reaction. The high electronegativite atoms or groups in the counterion are essential. Another crucial factor is bond strength between the central ion and the ligand. Studies of the N5Sb(OH)4F2 isomers indicate that the OH rather than the fluorine ions in the axial coordination positions could stabilize the decomposition transition structure through partial dissociation of the Sb–OH bond, which is used to explain the reason why there is an obvious difference in the decomposition activation enthalpies of the three isomers.
Introduction
Polynitrogen compounds have received great attention as high energy density materials (HEDMs) for propulsion or explosive applications.1–7 Although a wealth of theoretical calculations have indicated that Nn (n = 4, 6, 8, 11) systems may exist in acyclic forms or linear structures,8–14 no such compounds have yet been successfully synthesized15,16 because of their extreme instability. Inspiringly, N5AsF6 was successfully synthesized in 1999 (ref. 17) by Christe, which caused an instant sensation and shocked the world. Indeed, N5AsF6 was considered as only the third known compound containing a stable homoleptic polynitrogen moiety, after N2 isolated in 1772 by Rutherford and N3− first synthesized in 1890 by Curtius.18 However, this white solid was only marginally stable at 22 °C and the damage it caused during low temperature Raman analysis revealed its instability. In 2001, N5SbF6 (ref. 19) was successfully obtained and proved to be more stable, decomposing only at above 70 °C.To date, a total of 12 N5+-containing salts have been synthesized,17,20,21 among which N5SbF6 is the most stable. In more than a decade, no further novel N5+-containing salts with better thermal stability have been synthesized. Most published papers on N5+-containing salts focused on their synthesis and properties, whereas there seems to have been limited research on the mechanism of their thermodynamic stability.22,23 Questions arise, such as why N5SbF6 has quite high thermal stability. In order to gain insight into the role of the central Sb atom as well as of the ligand F atoms in SbF6−, other N5+M− salts (M = Sb(OH)6, SbF2(OH)4, BF4, B(CF3)4, AlF4, AlF6, AsF6, and PF6) (Scheme 1) have been selected for a comparative study.
Computational details
All calculations were performed using the Gaussian 09 package.24 Geometry optimizations of the starting structures were carried out at the M06-2X (ref. 25) level with the 6-311+G(d) basis set for B, C, N, F, Al and P atoms and SDD for other heavy metal atoms.22 Each optimized structure was confirmed as local energy minima on the potential-energy surface without imaginary frequencies. Note that M06-2X functional is very suitable to multi-nitrogen or pure nitrogen molecules according to previous studies.26Thermal stability is a key factor in evaluating the synthetic possibilities, and is closely related to the activation enthalpy of thermal dissociation (ΔH≠), which is determined by the transition state (TS). Calculations were performed to obtain the barrier heights in order to further discuss the relative stability of the relevant compounds. Activation enthalpy (ΔH≠) was calculated according to eqn (1):
| ΔH≠ = ΔHTS − ΔHR | (1) |
Results and discussion
Geometries of N5+M−
The structure of N5SbF6 (see structure 2 in Fig. 1) was in good agreement with the reported data,23 which proved that our employed method was reliable. The obtained structures of N5+-containing salts 1 to 11 are depicted in Fig. 1 and 2, in which compounds 3, 4, 10, and 11a–11c are the novel designed complexes indicated in Scheme 1. Selected bond lengths are listed in the figures. | ||
| Fig. 1 Geometrical structures of the N5+ complexes in which the central atom Sb was substituted by Al, P, B, As and Sn with selected bond length shown in the structures. | ||
 | ||
| Fig. 2 Geometrical structures of the N5+ complexes in which the ligand F was substituted by OH and CF3 in N5SbF6 and N5BF4 with selected bond length shown in the structures. | ||
The structural difference between the compounds 3, 4 and 5–8 in Fig. 1 is that they have the same ligand F but different central atoms, P, Al, As and Sn, respectively. The four compounds 2, 11a, 11b, and 11c in Fig. 2 have the same central atom Sb, but four or six of the fluorine atoms in N5SbF6 are substituted by OH. Compounds 11a, 11b, and 11c are structural isomers with the same C2 point group. They differ in the positions of the two fluorine atoms of the counter-ion. From the bond length shown in Fig. 1 and 2, it is noteworthy that the bond lengths in the isolated N5+ unit are slightly longer than those in the other N5+ salts, except in the case of structure 3 (N5AlF6), in which the N(1)–N(3) bond length (1.311 Å) is longer than that in the isolated N5+ unit (1.300 Å). These data showed that the central Al ion is strongly bonded with only four fluorine anions, while the other two fluorine ions are clearly away from the central Al ion through observation of the Al–F bond lengths of 1.784–1.961 Å in 3 (N5AlF6) and 1.664–1.727 Å in 4 (N5+AlF4−). These indicate the stronger Al–F bonds in the latter. Additionally, the Sb(6)–F(7) and Sb(6)–F(8) bond lengths (1.959 Å) in N5SbF6 are clearly shorter than the corresponding Sb–F bond lengths (2.002 Å) in 11a. The similar cases are also observed through comparison of the bond lengths between Sb(6)–F(9) in 2 and Sb(6)–F(7) in 11b, Sb(6)–F(10) in 2 and Sb(6)–F(7) in 11c. Combined with N5+SbF6− results, N5+ with a high oxidation potential influences the Sb–F bond length. Moreover, the numbers of fluorine in counterion clearly influence the electronegativity, which results in the difference of Sb–F bond length. These structural differences could reflect the variation of stability of the Sb-containing N5+ salts.
Decomposition mechanism of N5+M−
For the thermal decomposition of isolated N5+, the activation enthalpy was estimated to be 47.2 kcal mol−1, indicating that N5+ has a good thermal stability. In addition, we explored the electronic spin reverse, i.e. the photodissociation and coupling of thermal cracking events that may occur during the thermal dissociation of the N5+ cation. The energy curves for both the triplet and singlet states of N5+ are shown in Fig. 3. As the reaction coordinate changes, there is a crossover point between the two states of N5+. The actual thermal dissociation process should initiate from the reactant to the crossover point; thereafter, this process continues along the triplet potential energy surface of N5+ to the products, the linear triplet N3+ and N2, as suggested by Nguyen.5 The energy difference between the crossover point and the reactant was estimated to be ca. 35.6 kcal mol−1. The above analysis indicates that the N5+ is thermally stable in the gas phase.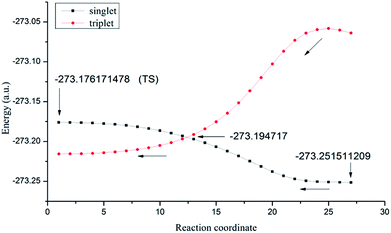 | ||
| Fig. 3 The energy curves for both the triplet and singlet states of N5+ during its dissociation process. | ||
When N5+ is combined with M−, the decomposition mechanism of N5+ should be similar to that in its isolated case. Table 1 showed NPA charge plus of all nitrogen atoms of N5+ salts in the reactant and their decomposition transition states.
| a qN (a) represents the NPA charge plus of all nitrogen atoms of each salt in the reactant state. qN (b) represents the NPA charge plus of all nitrogen atoms of each salt in the decomposition transition state. | ||||
|---|---|---|---|---|
| Compound | N5AlF4 | N5AlF6 | N5AsF6 | N5SbF6 |
| qN (a) | 0.958 | 0.756 | 0.961 | 0.964 |
| qN (b) | 0.783 | 0.386 | 0.786 | 0.785 |
| Compound | N5BF4 | N5B(CF3)4 | N5PF6 | N5Sb(OH)6 |
| qN (a) | 0.952 | 0.970 | 0.959 | 0.941 |
| qN (b) | 0.744 | 0.789 | 0.778 | 0.624 |
| Compound | 11a | 11b | 11c | |
| qN (a) | 0.940 | 0.952 | 0.942 | |
| qN (b) | 0.664 | 0.718 | 0.645 | |
From Table 1 we can find the partially negative charges (ca. 0.03–0.05e) transfer from the counterion to N5+ of the salt in the reactant states. N5AlF6 has the most amount of charge transfer. These results discover the intermolecular essentially electrostatic interaction between M− and N5+. Moreover, this interaction can further induce N5+ decomposition into N2 and more oxidative N3+, which results in more negative charge transfer from the counterion to the N2⋯N3+ complex, seen in Table 1. Ultimately, N3+ can capture one F anion of the counterion to form N3F product (seen in ESI†). Thus, the stronger the ability of M− to retain electrons in the decomposition reaction, the more stable the N5+M− species is.
The role of the central atom in stabilizing N5+M−
To study the role of the central atom in stabilizing N5+, we replaced Sb in N5SbF6 with Al, B, P and As. Table 2 lists the thermal decomposition activation enthalpy (ΔH≠) and reaction enthalpy (ΔrH) of the N5+-containing salts. Calculations show that the thermal stability is in the order N5B(CF3)4 ≈ N5SbF6 ≈ N5AsF6 > N5PF6 > N5AlF4 ≈ N5BF4 > N5AlF6. On one hand, the larger volume of the counterion favors improvement of the thermal stability of the salt. On the other hand, the present reactivity order is clearly explained through the amount of the negative charge transfer from the counterion to N5+ during the reaction procedure, seen Table 1. An interesting case is found for N5AlF6, where the two fluorine ions in the equatorial positions are obviously separated from the central Al ion. The partially dissociated Al–F bond suggests that the two F ions could be not strongly bound by the central Al ion so that F ion tends to shift to N5+, which could promote decomposition of the N5+ cation. The shorter Al–F bond in N5AlF4 indicates stronger interaction between Al and F, which is relatively more difficult to be broken in the decomposition reaction. This is why AlF4− should be preferred over AlF6− to stabilize N5+. Both activation and reaction enthalpies calculations indicated that N5AlF4 is more stable than N5AlF6, which is consistent with the conclusion that the bond strength between the central atom and the ligand is significant for N5+ stability. The more strongly bound F anion in SbF6− and AsF6− has a relatively less chance for dissociation of F anion so as to approach N5+, which leads to their higher thermal stability. Moreover, comparison of thermodynamics between N5BF4 and N5B(CF3)4 salts shows that group B(CF3)4− with higher electronegativity should be preferred to stabilize N5+. Note that the reaction enthalpy of N5SbF6 is only small positive among the salts, which shows that the salt has not only dynamic stability but thermodynamic one. Though N5PF6 has the barrier height close to N5SbF6, more exothermic decomposition reaction of N5PF6 could be one of the reasons why the salt is not stable enough in room temperature.| Compound | N5AlF4 | N5AlF6 | N5AsF6 | N5SbF6 |
| ΔH≠ | 27.1 | 8.5 | 31.5 | 31.6 |
| ΔrH | −4.7 | −56.9 | −2.5 | +1.2 |
| Compound | N5BF4 | N5B(CF3)4 | N5PF6 | |
| ΔH≠ | 27.1 | 34.1 | 30.5 | |
| ΔrH | −17.5 | −0.2 | −10.9 |
The role of fluorine atoms in stabilizing N5+M−
All known stable N5+-containing salts have F atoms in their counterions. To better study the role of fluorine ion in stabilizing N5+M−, the OH group was selected to substitute F because OH and F are isoelectronic and moreover, oxygen and fluorine have the closest electronegativities among all single atoms. The calculated activation energy of N5Sb(OH)6 is 19.1 kcal mol−1, which is much lower than that of N5SbF6 (30.3 kcal mol−1).This large thermal stability difference between N5Sb(OH)6 and N5SbF6 should originate from the different electronegativity of fluorine and OH. Fluorine has higher electronegativity than OH, and for the same central atom Sb, the bond strength of Sb–F is greater than that of Sb–OH. Thus, electrons are more favorably bound in SbF6− and less charge is transferred to N5+ to accelerate its dissociation, compared with the case in N5Sb(OH)6. Considering that fluorine has the highest electronegativity of any single atom, we may reasonably infer that fluorine is indispensable in stabilizing N5+-containing salts if the ligand is a single atom. Additionally, seeking other potential functional groups with high electronegativity contained in the counterion might be helpful to improve the stability of the N5+ salts.
There are six fluorine ions in SbF6−, and a further question that arises is which of these plays a major role in stabilizing N5+. In order to ascertain the position of the functional fluorine ions, we studied the N5Sb(OH)4F2 isomers – 11a, 11b, and 11c. Calculations show that the relative enthalpy of the above isomers is 0.0 (11a), 0.8 (11b), and 0.3 (11c) kcal mol−1 in their reactant states. However, the relative enthalpy is 0.0 (11a), 7.7 (11b), and −0.6 (11c) kcal mol−1 in their transition states. The order of their activation barriers is 11b (28.6) > 11a (21.7) > 11c (20.8). These data clearly show that the stability variation among the isomers should be attributed to the difference of their total energies in the transition states. NBO calculations show that in the decomposition transition structure of 11b, there are the smallest charge amounts transferred from the counterion to N5+ decomposition structure, which corresponds to the highest reaction barrier height among the decomposition reactions of the three isomers. Actually, the two fluorine atoms in the axial coordination positions of 11b are relatively not favorable to stabilize the transition structure. Comparison of the transition structures of the three isomers discovers that the two OH ions in the axial coordination positions of 11a and 11c should be better selection to stabilize the transition structures with respect to lower barrier heights. The case should be attributed to the stronger Sb–F than Sb–OH bonds so that the former is relatively more difficult to be ruptured to form F ion. Thus, the bound F has no enough negative charge on itself to stabilize the formed N3+ fragment in the transition structure. In 11a and 11c, one of the axial coordination OH anions could stabilize the N3+ fragment in the transition structure through Sb–OH partial bond dissociation. The remaining two fluorine ions should also contribute to stabilize N5+, whether they are close to or far away from the formed N5+ fragment. This is consistent with the conclusion of 11a and 11c having almost the same barrier heights.
Conclusions
Density functional theory studies have indicated that the interaction between M− ion and the N5+ cation causes thermal instability in the latter. Strong bonding between the central atom and the ligand atom in the counter anion is significant for holding ligand anion in M− so as to favour N5+ stability. Among the simple single-atom ligands, fluorine is the best choice due to its highest electronegativity. In addition, fluorine atoms in specific positions show greater contributions to control the N5+ decomposition. We hope that our theoretical work could provide a useful contribution for future synthesis of the novel and more complex anions to stabilize N5+.Acknowledgements
The authors gratefully acknowledge the support of the Foundation of NSAF (No. 11176004).References
- F. Cacace, Chem.–Eur. J., 2002, 8, 3838–3847 CrossRef CAS.
- G. Chung, M. W. Schmidt and M. S. Gordon, J. Phys. Chem. A, 2000, 104, 5647–5650 CrossRef CAS.
- M. I. Eremets, R. J. Hemley, H.-k. Mao and E. Gregoryanz, Nature, 2001, 411, 170–174 CrossRef CAS PubMed.
- S. Fau and R. J. Bartlett, J. Phys. Chem. A, 2001, 105, 4096–4106 CrossRef CAS.
- M. T. Nguyen and T.-K. Ha, Chem. Phys. Lett., 2001, 335, 311–320 CrossRef CAS.
- G. A. Olah, G. K. Surya Prakash and G. Rasul, J. Am. Chem. Soc., 2001, 123, 3308–3310 CrossRef CAS PubMed.
- W. E. Thompson and M. E. Jacox, J. Chem. Phys., 1990, 93, 3856–3862 CrossRef CAS.
- T. M. Klapötke, J. Mol. Struct.: THEOCHEM, 2000, 499, 99–104 CrossRef.
- Q. S. Li, L. J. Wang and W. G. Xu, Theor. Chem. Acc., 2000, 104, 67–77 CrossRef CAS.
- Q. S. Li and J. F. Zhao, J. Phys. Chem. A, 2002, 106, 5928–5931 CrossRef CAS.
- Y. D. Liu, P. G. Yiu, J. Guan and Q. S. Li, J. Mol. Struct.: THEOCHEM, 2002, 588, 37–43 CrossRef CAS.
- Y. D. Liu, J. F. Zhao and Q. S. Li, Theor. Chem. Acc., 2002, 107, 140–146 CrossRef CAS.
- M. Tobita and R. J. Bartlett, J. Phys. Chem. A, 2001, 105, 4107–4113 CrossRef CAS.
- L. J. Wang, P. Warburton and P. G. Mezey, J. Phys. Chem. A, 2002, 106, 2748–2752 CrossRef CAS.
- F. Cacace, G. de Petris and A. Troiani, Science, 2002, 295, 480–481 CrossRef CAS PubMed.
- L. Gagliardi, S. Evangelisti, P.-O. Widmark and B. O. Roos, Theor. Chem. Acc., 1997, 97, 136–142 CrossRef CAS.
- K. O. Christe, W. W. Wilson, J. A. Sheehy and J. A. Boatz, Angew. Chem., Int. Ed., 1999, 38, 2004–2009 CrossRef CAS.
- T. Curtius, Ber. Dtsch. Chem. Ges., 1890, 23, 3023–3033 CrossRef.
- A. Vij, W. W. Wilson, V. Vij, F. S. Tham, J. A. Sheehy and K. O. Christe, J. Am. Chem. Soc., 2001, 123, 6308–6313 CrossRef CAS PubMed.
- R. Haiges, S. Schneider, T. Schroer and K. O. Christe, Angew. Chem., Int. Ed., 2004, 43, 4919–4924 CrossRef CAS PubMed.
- W. W. Wilson, A. Vij, V. Vij, E. Bernhardt and K. O. Christe, Chem.–Eur. J., 2003, 9, 2840–2844 CrossRef CAS.
- M. T. Nguyen and T.-K. Ha, Chem. Phys. Lett., 2000, 317, 135–141 CrossRef CAS.
- F. Wang, H. Du, J. Zhang and X. Gong, Struct. Chem., 2011, 22, 1067–1073 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision A.1, Gaussian, Wallingford, Conn, USA, 2009 Search PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- C. Qi, R.-b. Zhang and S.-p. Pang, RSC Adv., 2013, 3, 17741–17748 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16304h |
| This journal is © The Royal Society of Chemistry 2015 |

