Corrosion and tribocorrosion performance of multilayer diamond-like carbon film in NaCl solution
Abstract
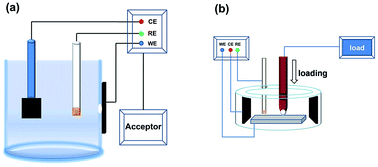
The anticorrosion and tribocorrosion properties of a multilayer diamond-like carbon (DLC) film were systematically investigated in NaCl solution. Electrochemical measurements suggest that the corrosion performance of the multilayer DLC film is superior to those of the substrate and single layer DLC film in NaCl solution, which is attributed to the successively multilayered structure with a well-bonded interface and the formation of Si oxides. An extremely high Warburg impedance value, higher than 107 Ω cm2, of the multilayer DLC film has been observed. Tribocorrosion tests show that the multilayer DLC film presents lower wear rate in NaCl solution, with the substrate and single layer DLC film as comparisons. We demonstrate that the multilayer DLC film is an excellent protective material for improving both corrosion and wear performance of the substrate.

 Please wait while we load your content...
Please wait while we load your content...