Morphology-controllable synthesis of novel Bi25VO40 microcubes: optical properties and catalytic activities for the reduction of aromatic nitro compounds
Lei Zhangab,
Jin-Song Hua,
Cheng-Ling Pan*ab,
Xin-Hua Huang*a and
Chang-Min Houb
aLaboratory of Multiscale Materials and Molecular Catalysis, School of Materials Science and Engineering, Anhui University of Science and Technology, Huainan, Anhui 232001, P. R. China. E-mail: clpan@aust.edu.cn; hxh0317@hotmail.com
bState Key Lab of Inorganic Synthesis & Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 8th September 2015
Abstract
In the present paper, novel Bi25VO40 microcubes with relatively good dispersion have been fabricated via a facile, fast and mild hydrothermal synthesis strategy. The morphology and compositional characteristics of the Bi25VO40 sample were characterized by various testing techniques including powder X-ray diffraction (XRD), inductively coupled plasma-atomic emission spectroscopy (ICP-AES), FT-IR spectroscopy, energy dispersive spectrometry (EDS), X-ray photoelectron spectroscopy (XPS), high resolution transmission electron microscopy (HRTEM) and scanning electron microscopy (SEM). Several experimental parameters including synthesis temperature, time and the amount of additives (e.g., polyacrylamide, sodium citrate and sodium hydroxide) were discovered to play vital roles in the construction of these Bi25VO40 microcrystals. From the UV-vis diffuse reflectance spectrum (DRS) of the Bi25VO40 its band gap was calculated to be 2.42 eV. The photoluminescence (PL) spectrum showed that Bi25VO40 microcubes had two obvious emission peaks centered at 458 and 639 nm. Moreover, catalytic experiments showed that the as-prepared Bi25VO40 microcubes possessed superior catalytic performance for the conversion of 4-nitrophenol (4-NP) and 4-nitroaniline (4-NA) in the presence of excess NaBH4 solution.
1. Introduction
During the past decade, the controllable preparation of nano- or micro-scale metal oxides with uniform dimensions and shapes has attracted considerable interest, because the physical and chemical properties of materials depend highly on their sizes and shapes.1–10 Therefore, morphology-controllable synthesis of novel and well-defined metal oxide micro/nanostructures has been one of the hot areas in the field of chemistry and material research.11–15 Recently, studies further demonstrate that ternary oxides usually possess better performances than binary counterparts in many cases such as optical, electronic, magnetic, mechanical, and piezoelectricity properties due to their stoichiometric diversity and structural richness.16–20 However, phase and morphology control of these complex metal oxides with a desired chemical composition is still a big challenge up to now because of their various stoichiometries (e.g., typical chemical formulas of ABO4, A4B2O11, A25BO40, A46B8O89 and so on) and the associated complex structures.21–27Bismuth vanadates are an important class of Bi-based ternary metal oxides. Usually, there are many phases existed in the well-known Bi–V–O family including BiVO4, Bi4V2O11, B25VO40, Bi46V8O89 and so on.24–27 In addition, it has showed that these kind complex metal oxides exhibit excellent oxide ion conductivity, photocatalytic, dielectric, ferroelectric and pyroelectric properties.28–30 Therefore, controllable fabrication of these functional micro/nanomaterials with various morphologies is very significant from the perspective of the fundamental research and technological application. Actually, subsequent studies have proved this point. For example, Xing's research team prepared hierarchically structural BiVO4 photocatalysts with special morphologies through a facile one-pot method in the presence of soluble starch.31 Kuang and coworkers synthesized a new polymorph of bismuth vanadate Bi46V8O89 with excellent ionic conductivity by a high temperature solid state reaction.25 Chen et al. reported the successful preparation of hierarchical Bi4V2O11 hollow microspheres with excellent visible activities by a facile template-free solvothermal route.32 Besides the above three materials, other bismuth vanadates with different atomic ratio of Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O such as Bi25VO40, has been discovered years ago. Researches show that Bi25VO40 belongs to the sillenite group with a pseudo-body centered cubic unit cell and the noncentrosymmetric space group I23 and it usually exhibits unique photorefractive, photochromic, electrooptic and dielectric properties.33–38 Therefore, realization of the morphology-controllable synthesis of Bi25VO40 micro/nanomaterials not only expects to obtain some novel nanostructures and enriches the preparation technology of nanomaterials, but also has great significance for the performance modulation and optimization of Bi25VO40 materials. Unfortunately, to the best of our knowledge, report about the fabrication of Bi25VO40 micro/nanostructures still remains rare, especially for Bi25VO40 microcubes.
O such as Bi25VO40, has been discovered years ago. Researches show that Bi25VO40 belongs to the sillenite group with a pseudo-body centered cubic unit cell and the noncentrosymmetric space group I23 and it usually exhibits unique photorefractive, photochromic, electrooptic and dielectric properties.33–38 Therefore, realization of the morphology-controllable synthesis of Bi25VO40 micro/nanomaterials not only expects to obtain some novel nanostructures and enriches the preparation technology of nanomaterials, but also has great significance for the performance modulation and optimization of Bi25VO40 materials. Unfortunately, to the best of our knowledge, report about the fabrication of Bi25VO40 micro/nanostructures still remains rare, especially for Bi25VO40 microcubes.
Hence, we provide a facile hydrothermal method to the synthesis of novel Bi25VO40 microcubes. Some experimental parameters affecting the phase and morphology of the final sample are studied in detail. In addition, the optical and catalytic performances of Bi25VO40 sample are investigated. Several distinguishing features are presented below: (1) novel Bi25VO40 microcubes are obtained; (2) the preparation strategy is relatively facile, mild and fast; (3) no post-treatment is required; (4) the Bi25VO40 sample possesses superior catalytic reactivity towards the reduction of 4-nitrophenol and 4-nitroaniline in excess NaBH4 aqueous system.
2. Materials and methods
For the preparation of Bi25VO40 microcubes, Bi(NO3)3·5H2O, NH4VO3, Na3C6H5O7·2H2O, NaOH and polyacrylamide (PAM, molecular weight = 3 million) were employed as Bi source, V source, coordination agent, mineralizer and surfactant, respectively. In a typical synthesis process, 0.0012 mol of Bi(NO3)3·5H2O was added into 15 mL of deionized water followed by addition of 0.0024 mol of Na3C6H5O7·2H2O. Then, 15 mL of aqueous solution containing NH4VO3 (0.0001 mol), NaOH (0.3 g) and polyacrylamide (0.3 g) was slowly dropped into this reaction system under vigorous stir for 40 min. Finally, the as-obtained suspension was poured into a Teflon-lined oxidation-resisting steel autoclave, and kept at 150 °C for 2 h, then cooling to atmospheric temperature naturally. The precipitant was recovered by centrifugation, followed by washing several times to remove excess surfactants and residual ions, and drying at 80 °C for 8 h.XRD measurement was recorded on a Shimadzu XRD-6000 powder X-ray diffractometer in the 2θ range from 10° to 70°, with Cu Kα radiation (λ = 1.5418 Å). SEM image of the sample was obtained using a field emission scanning electron microananlyser (S-4800 from Hitachi Corp.), operated at an acceleration voltage of 5 kV. EDS, being attached to the SEM, was employed to measure the composition of the product. HRTEM was carried out on a JEM-2100 high resolution transmission microscope, employing an accelerating voltage of 200 kV. XPS data was collected on a thermo ESCALAB 250XI X-ray photoelectron spectrometer. FT-IR spectrum was obtained for KBr-diluted sample on a Nicolet Magna 750 IR spectrometer in the range of 400–1000 cm−1. The UV-vis DRS of the product was collected with a UV-vis spectrophotometer (UV-4100 from Hitachi Corp.). PL spectrum was recorded on an Edinburgh FLSP 920 fluorescence spectrophotometer at room temperature. The Bi/V atomic proportion of the sample was determined by ICP-AES (Profile Spec from Leeman Corp.).
To assess the catalytic ability of Bi25VO40 microcubes for the transformation of 4-NP to 4-aminophenol (4-AP), some solutions that were utilized in the reaction need to be prepared freshly. The catalytic experiments were actualized as follows: 1.0 × 10−4 mol L−1 of 4-NP and appropriate amount of Bi25VO40 catalysts were first mixed; a certain volume of NaBH4 solution was then added into the above reaction system to give total 4 mL of the solution. The reduction process was monitored by UV-vis spectrophotometer (UV-9000S, metash, China). For the reduction reaction of 4-NA, 1.0 × 10−4 mol L−1 of 4-NP was replaced by 2.0 × 10−4 mol L−1 of 4-NA, while keeping other experimental conditions constant.
3. Results and discussion
The phase and purity of the Bi25VO40 microcubes was first checked by XRD analysis. Fig. 1a shows the experimental XRD pattern of the sample synthesized at 150 °C for 2 h. Every diffraction peak can be readily assigned to the standard Bi25VO40 material with a cubic phase, which is in accordance with the reported data (JCPDS file Card no. 46-0419). The intensity and shape of the peaks reveal that the Bi25VO40 sample is well crystallized. No other diffraction peaks for impurity phases is found from the XRD pattern. The vibration spectrum of Bi25VO40 sample was further analyzed through FT-IR spectrum (Fig. 1b). The two peaks at 475 and 763 cm−1 are contributed by the bending and stretching vibration of VO43−, respectively.39,40 The peak centered at 525 cm−1 corresponds to the stretching vibration of Bi–O–V, and a weak peak at 600 cm−1 is attributed to the vibration of Bi–O bond.41 Therefore, it is reasonable to believe that the pure Bi25VO40 product has been successfully prepared via the hydrothermal synthesis strategy. | ||
| Fig. 1 XRD pattern (a) and FT-IR spectrum (b) of the as-obtained Bi25VO40 microcubes prepared at 150 °C for 2 h. | ||
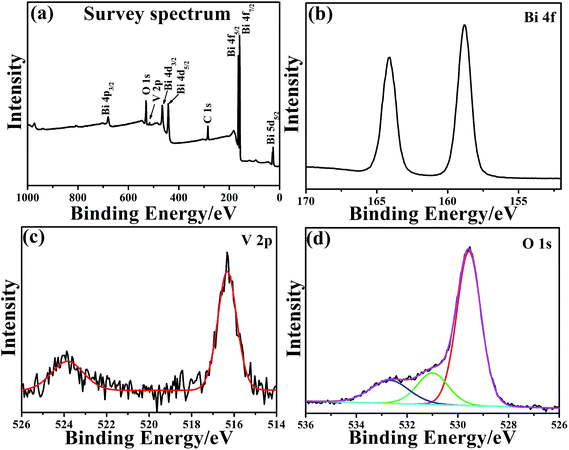
More detailed surface compositions and chemical states of the elements in Bi25VO40 sample were revealed by XPS (Fig. 2). Besides the Bi, V and O signals deriving from Bi25VO40 sample, the C 1s peak centered at 284.8 eV, which is considered to be added in the sample preparation process, can also be found and employed as a binding energy reference, as shown in Fig. 2a. The narrow spectrum of Bi 4f is shown in Fig. 2b, which possesses two Bi 4f signals at about 158.8 eV (Bi 4f7/2) and 164.1 eV (Bi 4f5/2), indicating that Bi is in the Bi3+ oxidation state.30 As for the V 2p shown in Fig. 2c, the peaks at 523.8 and 516.3 eV can be accordingly attributed to the V 2p1/2 and V 2p3/2, which is the characteristic of the V species with a +5 valence in Bi25VO40.42 In Fig. 2d, the O 1s peak can be separated into three signals at 529.6, 531.0 and 532.6 eV, which should be ascribed to the binding energies of O in the V–O bond, Bi–O bond and hydroxyl (or crystal water) adsorbed on the surface, respectively.43 Moreover, ICP-AES technology was employed to analyze the atomic proportion of Bi/V in the final sample. Experiment indicates that this value is about 24.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is highly consistent with the stoichiometric ratio of Bi25VO40, indicating that the final sample is comprised of pure Bi25VO40 sample.
1, which is highly consistent with the stoichiometric ratio of Bi25VO40, indicating that the final sample is comprised of pure Bi25VO40 sample.
The morphology and size of the Bi25VO40 sample was investigated by SEM. As displayed in Fig. 3a, it is easily found that the final product is comprised of many Bi25VO40 microcubes with uniform size and shape. High magnification SEM image displayed in Fig. 3b indicates that the whole microcubes have smooth faces and the edge-length is around 1–2 μm. EDS elemental mapping of Bi25VO40 sample also describes the space distribution of Bi, V and O in an individual microcube (Fig. 3c–f), which shows that three elements are evenly distributed in the cube-like structure.
The morphology of the sample was revealed to be seriously influenced by the synthesis temperature. If the synthesis temperature was set at 120 °C, keeping other experimental conditions unchanged, micro-scale cubes were readily obtained (Fig. 4a). However, high magnification SEM, TEM images and the size distribution histogram of the sample displayed in Fig. 4b–d shows that many polydisperse nanoparticles with wide size distribution (32–42 nm) attach to the surface of cube-like microstructures. When the temperature was enhanced to 150 (Fig. 3) or 180 °C (Fig. 4e), the final product was comprised of uniform microcubes. Furthermore, it should be noted that all the XRD patterns of these three samples can be indexed to pure Bi25VO40 with cubic phase (Fig. 1 and 5). Therefore, it is reasonable to believe that Bi25VO40 nanoparticles may first form during the synthesis process. At higher reaction temperature, such particle-like precursors gradually dissolve and more Bi25VO40 microcubes with larger size are obtained. Actually, this phenomenon can be supported by well-known Ostwald ripening mechanism.44
 | ||
| Fig. 4 SEM, TEM images and the size distribution histogram of the samples prepared at different reaction temperature: (a–d) 120 and (e) 180 °C. | ||
It has demonstrated that the amount of sodium hydroxide in the reaction system can significantly influences the shape and phase of the sample. When the amount of sodium hydroxide was controlled at 0.15 g, amorphous agglomerate particles were produced (Fig. 6a and b and 7, down). While increasing the amount of sodium hydroxide to 0.3 (Fig. 1 and 3) or 0.45 g (Fig. 6c and 7, middle), monodisperse Bi25VO40 microcubes were obtained. Further increasing the amount of sodium hydroxide to 0.6 g, microrods and microcubes survived together in the sample (Fig. 6d and e) and the corresponding XRD pattern displayed in Fig. 7 (upper) revealed that the sample was comprised of two phase, which could be assigned to cubic Bi25VO40 (no. 46-0419) and orthorhombic BiVO4 (no. 12-0293), respectively. As a conventional mineral reagent, the presence of appropriate amount of sodium hydroxide in the hydrothermal or solvothermal process can remarkably speed up the reaction rate, which is in favor of the nucleation and growth of target product.45 In our experiment, the lower amount of sodium hydroxide means the slower formation rate of Bi25VO40, which may take responsibility for the appearance of amorphous agglomerate particles. However, excess sodium hydroxide in the reaction medium usually leads to the generation of some impurities, which has been adequately demonstrated in the preparation of other complex metal oxides nanomaterials such as BiFeO3, Cd2Ge2O6, Bi4Ge3O12 and so on.46–48 Therefore, in order to prepare pure Bi25VO40 sample, the optimal amount of sodium hydroxide should be controlled at 0.3 g.
 | ||
| Fig. 6 SEM and TEM images of the samples with different amount of sodium hydroxide: (a and b) 0.15, (c), 0.45 and (d and e) 0.6 g. | ||
During the current hydrothermal process, we found that the organic macromolecule PAM also has a crucial effect on the morphology and dispersity of Bi25VO40 sample. In the absence of PAM, the as-prepared sample was composed of agglomerate cube-like Bi25VO40 microstructures (Fig. 8a and b), while other conditions keeping the same with the typical synthesis. If the amount of PAM was set at 0.3 (Fig. 1 and 3) or 0.6 g (Fig. 8c and d), uniform Bi25VO40 microcubes were the exclusive product. On one hand, the viscosity of the solution becomes greater due to the presence of PAM, which can effectively slow down the reaction rate. On the other hand, the amide groups of PAM molecule can coordinate with metal ions to generate stable coordination compound, and further adjust the reaction rate. Therefore, it is reasonable to believe that the improvement of agglomerating phenomenon can be attributed to the effective control of reaction rate resulting from the above double effect of PAM molecule.49
 | ||
| Fig. 8 SEM images and XRD patterns of the samples with different amount of PAM: (a and b) 0 and (c and d) 0.6 g. | ||
The amounts of sodium citrate can also sharply affect the morphology and phase of the sample. Several controllable experiments were carried out to check the effect of the sodium citrate in the construction of Bi25VO40 microcubes. For example, polydisperse Bi25VO40 microcubes and some irregular particles were obtained in the absence of sodium citrate (Fig. 9a). The corresponding XRD pattern displayed in Fig. 9b demonstrates that the sample is composed of two phases with cubic Bi25VO40 (no. 46-0419) and Bi2O3 (no. 27-0052). Raising the amount of sodium citrate to 0.0024 mol, monodisperse Bi25VO40 microcubes were obtained (Fig. 1 and 3). While the amount of sodium citrate was set at 0.0042 mol, the morphology of the sample hardly changed (Fig. 9c). However, the XRD pattern of this sample reveals that the BiVO4 impurity also exists in the system (Fig. 9d). As is well known, sodium citrate is a common coordination agents, which can reacts with Bi3+ to form a stable metal complex.50 Due to the coordination and dissolution equilibrium, the appropriate amounts of sodium citrate may sharply affect the concentration of free metal ions in the reaction system and further adjust the reaction and diffusion rate of the reagents, which may be responsible for the formation of uniform Bi25VO40 microcubes.
 | ||
| Fig. 9 SEM images and XRD patterns of the samples with different amount of Na3C6H5O7·2H2O: 0 (a and b) and 0.0042 mol (c and d). | ||
It has been demonstrated that the morphology of the obtained precursors can be easily adjusted by the reaction time. Fig. 10 represents the SEM images and XRD patterns of the samples synthesized at various reaction periods of 1, 2 and 12 h, respectively. As displayed in Fig. 10a and b, one can find that the final sample was composed of Bi25VO40 microcubes and nanoparticles when the reaction time was controlled at 1 h. Extending the reaction time to 2 h, nanoparticles gradually disappeared and more microcubes were obtained (Fig. 1 and 3). If the synthesis time was set at 12 h, the shape and phase of the sample remained unchanged (Fig. 10c and d). Therefore, the possible formation mechanism of the Bi25VO40 microcubes may be attributed to the distinguished Ostwald ripening process.44
 | ||
| Fig. 10 SEM images and XRD patterns of the samples obtained at 150 °C with different reaction time: 1 (a and b) and 12 h (c and d). | ||
The optical absorption of the as-obtained Bi25VO40 microcubes was investigated. Fig. 11 depicts the typical UV-vis DRS of the Bi25VO40 sample. For a crystalline semiconductor, the optical absorption near the band edge follows the equation αhν = A(hν − Eg)n/2, where α, h, ν, A and Eg are the absorption coefficient, Planck constant, light frequency, a constant and band gap, respectively. Also, n can be a value of 1 for the direct band gap and 4 for the indirect band gap.35 Therefore, from the long wavelength extrapolation of the band edge (Fig. 11b), the band gap of the Bi25VO40 micorcubes can be calculated to be 2.42 eV.
 | ||
| Fig. 11 DRS spectrum (a) and the plots of (αhν)2 vs. photo energy for the estimation of Eg of Bi25VO40 microcubes prepared at 150 °C for 2 h (b). | ||
Fig. 12 shows the excitation and emission spectra of the as-obtained Bi25VO40 microcubes at 150 °C for 2 h. In the present work, Bi25VO40 sample exhibits double obvious emission peaks at 458 and 639 nm with excitation wavelength at 360 nm (Fig. 12b). Actually, the origins of the above two PL emission peaks (458 and 639 nm) in Bi25VO40 sample have not been established up to now. However, recent researches have demonstrated that there are many oxygen vacancies existed in such sillenite group complex oxides.33–35 Therefore, it is reasonable to believe that the peak centered at 458 nm can be ascribed to oxygen vacancies related defects emission, which has been intensively studied in other metal oxides including ZnO and SnO2.51,52 The emission peak centered at 632 nm may be attributed to the presence of energy levels located in the bandgap, known as deep donors, which is in good agreement with the previous research report.53
 | ||
| Fig. 12 Room temperature excitation (a) and emission spectra (b) of the as-prepared Bi25VO40 microcubes prepared at 150 °C for 2 h (λex = 360 nm). | ||
In order to assess the catalytic activities of Bi25VO40 microcubes, the reduction reaction of 4-NP to 4-AP in the presence of overabounded NaBH4 was employed as the model experiment. Generally, 4-NP exhibits a main peak (λ = 317 nm) and a weak peak (λ = 400 nm) in the UV-vis absorption spectrum (Fig. 13a).54 The absorption peak of 4-NP undergoes an immediate red-shift from 317 to 400 nm upon the addition of NaBH4, indicating the formation of 4-nitrophenolate ions.55 Researches have demonstrated that such intermediate hardly changes a few days later till catalysts are introduced.56 Thus, the changes in absorbance at λ = 400 nm can be chosen as a index to report the transformation from 4-NP to 4-AP. Fig. 13b–d depicts the UV-vis absorption spectra of the solution at various reaction periods in different concentrations of catalyst (10, 15, and 20 mg L−1 Bi25VO40 microcubes). Obviously, the absorbance at λ = 400 nm gradually decreases with extending of the reaction time, and a new peak at about λ = 300 nm emerges that indicates superior catalytic activity of the Bi25VO40 microcubes for the conversion from 4-NP to 4-AP. Fig. 13e displays the correlation between the reaction rate and the concentration of the catalyst. The reaction rate can be quickened with the raising of the concentration of the catalyst from 10 to 15 to 20 mg L−1. The concentration of NaBH4 is higher than that of 4-NP, the catalytic reaction is in more abidance by the pseudo-first order reaction kinetics. Therefore, such rate constant of the catalytic reactions is obtained from the equation: ln(C0/C) = kt, where the C0 and C represent the concentrations of 4-NP solution at time 0 and t, respectively, and k is on behalf of the rate constant.57 Fig. 13f is the reaction kinetics of 4-NP solution based on the information recorded in Fig. 13e. The rate constants in different concentrations of Bi25VO40 catalysts are calculated to be 0.033 (10 mg L−1 of Bi25VO40), 0.092 (15 mg L−1 of Bi25VO40) and 0.136 min−1 (20 mg L−1 of Bi25VO40). Fig. 14 depicts the conversion–time curves of 4-NP over different catalysts (Bi25VO40 sample prepared in the absence of PAM, see Fig. 8a; Bi25VO40 sample with 0.6 g of PAM, see Fig. 8c). It can be easily found that the catalytic efficiencies in the presence of the above two catalysts significantly reduced. The serious agglomeration phenomenon of the product may explain the lower catalytic ability of the Bi25VO40 sample prepared without PAM molecules. The high amount of PAM added in the synthesis process may form a protective layer in the outer surface of Bi25VO40 microcubes, which makes against the electron transfer, resulting in the bad catalytic ability (Bi25VO40 sample with 0.6 g of PAM).58 Although the catalytic conversion process from 4-NP to 4-AP over the Bi25VO40 catalyst is still unclear, it is reasonable to believe that BH4− and 4-NP can be firstly adsorbed by the Bi25VO40 catalyst. Then, the electron transfer from BH4− to 4-NP can be mediated by the catalyst surface, leading to the production of 4-AP.59 Further investigations reveal that the Bi25VO40 microcubes also have promising catalysis in the reduction of other aromatic nitro-compounds, such as 4-NA under the same experimental conditions (Fig. 15), indicating huge potential for the applications in industrial production. Moreover, compared with some previous reports (see Table 1), the present Bi25VO40 nanostructures also presented close or better catalytic activity for the reduction of 4-NP. In particular, against Ag and Au catalysts the present Bi25VO40 catalysts have lower cost, which is very important in practical application.
4. Conclusions
We have designed and synthesized morphology-controllable Bi25VO40 microcubes that have the edge-length of 1–2 μm through a hydrothermal strategy. General experimental parameters that probably affect the phase and shape of the sample have been investigated in details. By modulation of these experimental parameters, we are capable of creation of the Bi25VO40 microcubes as wanted morphologies that would be more promising for the application in catalysis reaction. Prepared Bi25VO40 microcubes with well-defined structures have the optical absorption and photoluminescence properties that have been measured and analyzed deeply. Importantly, we have demonstrated that the as-prepared Bi25VO40 is able to exhibit superior catalytic activities for the reduction of 4-NP and 4-NA in excess NaBH4 aqueous system. In comparison to the existing hydrothermal/solvothermal strategy for the formation of similar microcubes, our strategy is more versatile and promising in construction of Bi25VO40 nanoarchitecture, since it is more facile, rapid and mild reaction system.Acknowledgements
We gratefully acknowledge financial support from the National Natural Science Foundation of China (No. 21501002, 21301005 and 21201006) and the Natural Foundation of Anhui Province (No. 1308085QB34 and 1408085QB31).References
- M. J. Zhi, G. W. Zhou, Z. L. Hong, J. Wang, R. Gemmen, K. Gerdes, A. Manivannan, D. L. Ma and N. Q. Wu, Energy Environ. Sci., 2011, 4, 139 CAS.
- H. A. Macpherson and C. R. Stoldt, ACS Nano, 2012, 6, 8940 CrossRef CAS PubMed.
- C. J. Chen, R. K. Chiang and Y. R. Jeng, J. Phys. Chem. C, 2011, 115, 18142 CAS.
- X. Guan, J. W. Nai, Y. P. Zhang, P. X. Wang, J. Yang, L. R. Zheng, J. Zhang and L. Guo, Chem. Mater., 2014, 26, 5958 CrossRef CAS.
- T. Taniguchi, K. I. Katsumata, S. Omata, K. Okada and N. Matsushita, Cryst. Growth Des., 2011, 11, 3754 CAS.
- H. J. Wu, L. D. Wang, Z. Y. Shen and J. H. Zhao, J. Mol. Catal. A: Chem., 2011, 351, 188 CrossRef CAS.
- H. J. Wu, L. D. Wang, J. Q. Zhang, Z. Y. Shen and J. H. Zhao, Catal. Commun., 2011, 12, 859 CrossRef CAS.
- H. J. Wu and L. D. Wang, Catal. Commun., 2011, 12, 1374 CrossRef CAS.
- L. F. Liotta, H. J. Wu, G. Pantaleo and A. M. Venezia, Catal. Sci. Technol., 2013, 3, 3085 CAS.
- H. Wu, G. Pantaleo, G. D. Carlo, S. Guo, G. Marcì, P. Concepción, A. M. Veneziaa and L. F. Liotta, Catal. Sci. Technol., 2015, 5, 1888 CAS.
- D. W. Wang, Q. H. Wang and T. M. Wang, CrystEngComm, 2010, 12, 3797 RSC.
- J. R. Huang, L. Y. Wang, C. P. Gu, M. H. Zhai and J. H. Liu, CrystEngComm, 2013, 15, 7515 RSC.
- L. Zhang, Z. M. Cui, Q. Wu, D. Guo, Y. Xu and L. Guo, CrystEngComm, 2013, 15, 7462 RSC.
- W. D. Shi, S. Y. Song and H. J. Zhang, Chem. Soc. Rev., 2013, 42, 5714 RSC.
- Y. Li and W. J. Shen, Chem. Soc. Rev., 2014, 43, 1543 RSC.
- L. Li, Y. Q. Zhang, F. Shi, Y. J. Zhang, J. H. Zhang, C. D. Gu, X. L. Wang and J. P. Tu, ACS Appl. Mater. Interfaces, 2014, 6, 18040 CAS.
- J. J. Ding, W. H. Yan, S. Sun, J. Bao and C. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 12877 CAS.
- D. W. Lee and K. M. Ok, Inorg. Chem., 2014, 53, 10642 CrossRef CAS PubMed.
- T. Taniguchi, T. Watanabe, K. Katsumata, K. Okada and N. Matsushita, J. Phys. Chem. C, 2010, 114, 3763 CAS.
- J. Zhang, J. H. Bang, C. C. Tang and P. V. Kamat, ACS Nano, 2010, 4, 387–395 CrossRef CAS PubMed.
- L. Chen, S. F. Yin, R. Huang, Q. Zhang, S. L. Luo and C. T. Au, CrystEngComm, 2012, 14, 4217 RSC.
- Y. Hu, D. Z. Li, F. Q. Sun, H. B. Wang, Y. Q. Weng, W. Xiong and Y. Shao, RSC Adv., 2015, 5, 54882 RSC.
- Z. S. Liu, J. N. Niu, P. Z. Feng, Y. W. Sui and Y. B. Zhu, RSC Adv., 2014, 4, 43399 RSC.
- Y. L. Geng, P. Zhang and S. P. Kuang, RSC Adv., 2014, 4, 46054 RSC.
- X. J. Kuang, J. L. Payne, J. D. Farrell, M. R. Johnson and I. R. Evans, Chem. Mater., 2012, 24, 2162 CrossRef CAS.
- Y. T. Lu, Y. F. Pu, J. Wang, C. X. Qin, C. L. Chen and H. J. Seo, Appl. Surf. Sci., 2015, 347, 719 CrossRef CAS.
- Y. G. Wang, R. He, M. Yang, T. Wen, H. Zhang, J. Liang, Z. S. Lin, Y. X. Wang, G. B. Li and J. H. Lin, CrystEngComm, 2012, 14, 1063 RSC.
- J. Z. Su, L. J. Guo, S. Yoriya and C. A. Grimes, Cryst. Growth Des., 2010, 10, 856 CAS.
- Y. F. Sun, Y. Xie, C. Z. Wu and R. Long, Cryst. Growth Des., 2010, 10, 602–607 CAS.
- Wendusu, A. Shiraishi, N. Takeuchi, T. Masuia and N. Imanaka, RSC Adv., 2015, 5, 44886 CAS.
- X. Y. Xing, Y. X. Ma, J. Li, G. Y. Fan, H. F. Ding, X. M. Ma, L. Yang and G. X. Xi, CrystEngComm, 2014, 16, 10218 RSC.
- X. F. Chen, J. B. Liu, H. Wang, Y. L. Ding, Y. X. Sun and H. Yan, J. Mater. Chem. A, 2013, 1, 877 CAS.
- W. F. Yao, H. Wang, X. H. Xu, X. F. Cheng, J. Huang, S. X. Shang, X. N. Yang and M. Wang, Appl. Catal., A, 2003, 243, 185 CrossRef CAS.
- J. W. Tang and J. H. Ye, Chem. Phys. Lett., 2005, 410, 104 CrossRef CAS.
- D. F. Hou, X. L. Hu, Y. W. Wen, B. Shan, P. Hu, X. Q. Xiong, Y. Qiao and Y. H. Huang, Phys. Chem. Chem. Phys., 2013, 15, 20698 RSC.
- A. W. Sun, H. Chen, C. Y. Song, F. Jiang, X. Wang and Y. S. Fu, RSC Adv., 2013, 3, 4332 RSC.
- Z. Wan, G. K. Zhang, J. T. Wang and Y. L. Zhang, RSC Adv., 2013, 3, 19617 RSC.
- Z. Wan and G. K. Zhang, Sci. Rep., 2014, 4, 6298 CrossRef CAS PubMed.
- J. B. Liu, H. Wang, S. Wang and H. Yan, Mater. Sci. Eng., B, 2003, 104, 36 CrossRef.
- U. M. García-Pérez, S. Sepúlveda-Guzmán and A. Martínez-de la Cruz, Solid State Sci., 2012, 14, 293 CrossRef.
- B. Lal, S. K. Patro and S. Singh, J. Sol-Gel Sci. Technol., 2010, 56, 340 CrossRef CAS.
- D. D. Qin, T. Wang, Y. M. Song and C. L. Tao, Dalton Trans., 2014, 43, 7691 RSC.
- D. Z. Li, W. Z. Wang, D. Jiang, Y. L. Zheng and X. M. Li, RSC Adv., 2015, 5, 14374 RSC.
- X. F. Cao, L. Zhang, X. T. Chen and Z. L. Xue, CrystEngComm, 2011, 13, 1939 RSC.
- Q. F. Han, J. Zhang, X. Wang and J. W. Zhu, J. Mater. Chem. A, 2015, 3, 7413 CAS.
- L. Zhang, X. F. Cao, Y. L. Ma, X. T. Chen and Z. L. Xue, J. Solid State Chem., 2010, 183, 1761 CrossRef CAS.
- Z. Q. Li, L. Zhang and X. T. Chen, Mater. Charact., 2012, 71, 24 CrossRef CAS.
- L. Zhang, X. F. Cao, X. T. Chen and Z. L. Xue, CrystEngComm, 2011, 13, 2464 RSC.
- W. R. Lee, L. Piao, C. H. Park, Y. S. Lim, Y. R. Do, S. Yoon and S. H. Kim, J. Colloid Interface Sci., 2010, 342, 198 CrossRef CAS PubMed.
- D. K. Ma, S. M. Huang, W. X. Chen, S. W. Hu, F. F. Shi and K. L. Fan, J. Phys. Chem. C, 2009, 113, 4369 CAS.
- Y. H. Tong, Y. C. Liu, C. L. Shao, Y. X. Liu, C. S. Xu, J. Y. Zhang, Y. M. Lu, D. Z. Shen and X. W. Fan, J. Phys. Chem. B, 2006, 110, 14714 CrossRef CAS PubMed.
- F. Gu, S. F. Wang, M. K. Lu, G. J. Zhou, D. Xu and D. R. Yuan, J. Phys. Chem. B, 2004, 108, 8119 CrossRef CAS.
- A. E. Nogueira, E. Longo, E. R. Leite and E. R. Camargo, Ceram. Interfaces, 2015, 41, 12073 CrossRef CAS.
- Y. Ma, Y. H. Ni, F. Guo and N. N. Xiang, Cryst. Growth Des., 2015, 15, 2243 CAS.
- F. Guo, Y. H. Ni, Y. Ma, N. N. Xiang and C. Liu, New J. Chem., 2014, 38, 5324 RSC.
- L. Gao, R. Li, X. L. Sui, R. Li, C. L. Chen and Q. W. Chen, Environ. Sci. Technol., 2014, 48, 10191 CrossRef CAS PubMed.
- H. Q. Li, L. N. Han, J. Cooper-White and I. Kim, Green Chem., 2012, 14, 586 RSC.
- L. Zhang, X. F. Cao, X. T. Chen and Z. L. Xue, J. Colloid Interface Sci., 2011, 354, 630 CrossRef CAS PubMed.
- Z. Ji, X. Shen, G. Zhu, H. Zhou and A. Yuan, J. Mater. Chem., 2012, 22, 3471 RSC.
- J. Wang, X. B. Zhang, Z. L. Wang, L. M. Wang, W. Xing and X. Liu, Nanoscale, 2012, 4, 1549 RSC.
- N. Sahiner, N. Karakoyun, D. Alpaslan and N. Aktas, Int. J. Polym. Mater. Polym. Biomater., 2013, 62, 590 CrossRef CAS.
- C. J. Huang, J. L. Hu, W. J. Fan, X. Wu and X. Q. Qiu, Chem. Eng. Sci., 2015, 131, 155 CrossRef CAS.
- M. Nasrollahzadeh, M. Maham and S. M. Sajadi, J. Colloid Interface Sci., 2015, 455, 245 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |