Highly promising discrimination of various catecholamines using ratiometric fluorescence probes with intermolecular self-association of two sensing elements†
Yanisa Sanguansapa,
Vithaya Ruangpornvisutia,
Thawatchai Tuntulania,
Vinich Promarakb and
Boosayarat Tomapatanaget*a
aDepartment of Chemistry, Faculty of Science, Chulalongkorn University, Phyathai Road, Bangkok, 10330, Thailand. E-mail: tboosayarat@gmail.com; Fax: +662-2541309
bSchool of Materials Science & Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong, 21210, Thailand
First published on 8th September 2015
Abstract
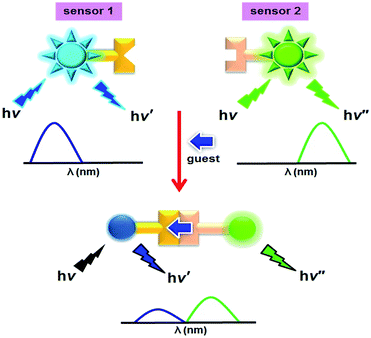
Two sensing elements based on fluorescence probes have been employed as the promising discriminating sensors of two catecholamines, dopamine (DA) and norepinephrine (NE), acting as a proper guest linker between two self-recognition sensing components. Surprisingly, in the presence of epinephrine (EPI), sensor NB containing boronic acid connected to a napthalimide unit demonstrated a very strong fluorescence enhancement while a large fluorescence quenching was observed in the case of DA and NE. To differentiate the structural similarity of DA and NE, an appropriately designed small fluorescence sensor CC containing a crown-ether attached to coumarin showed complementary recognition to an ammonium ion based catecholamine. The combination of NB and CC is capable of the differentiation of DA and NE with dual emission bands under a PET mechanism. The dual emission ratio (I475/I384) of the NB–DA–CC complex showed different values from those of the NB–NE–CC complex. Additionally, the PCA analysis using mixed sensors of NB and CC obviously separated DA and NE better than a single sensing element. This systematic approach is the first report showing a high potential for the identification of DA and NE using ratiometric fluorescence sensors with dual emission by two sensing elements.
Introduction
Catecholamine neurotransmitters, including dopamine (DA), norepinephrine (NE) and epinephrine (EPI), are a class of biogenic amines and have structural similarities as they consist of catechol and amino groups and play an important role as neurotransmitters involved in a variety of central nervous system functions.1,2 Generally, an unusual catecholamine concentration in body fluids is an essential indicator of Parkinson’s disease3,4 in clinical diagnosis. A critically elevated amount of EPI in human blood certainly implies heart failure and an increase in blood pressure.5 Importantly, a large amount of EPI and NE in the blood and urine possibly causes patients to suffer from adrenal gland tumors.6 Specific detection of each catecholamine species is a challenging task for sensing purposes. Detection of specific catecholamines, neurotransmitters, using artificial chemosensors with fluorescence spectroscopy7–11 which is a versatile technique with high sensitivity, rapid response, and easy execution, is currently of interest. Regarding the optical detection, there are a few reports concerning chemosensors for catecholamines,12 particularly containing boronic acid and aldehydes to covalently bind with the catechol and amine groups of the catecholamine species to form a boronate ester and an iminium ion, respectively.13 However, most fluorescence chemosensors previously reported the chelation-enhanced fluorescence quenching (CHEQ) effect upon binding with catecholamines.7 Concerning the discrimination of each catecholamine, many reports regarding fluorescence chemosensors showed non-specific binding among EPI, DA and NE.14 None of the fluorescence chemosensors for catecholamine sensing employed the Fluorescence Resonance Energy Transfer (FRET) process as a tool in detection. Interestingly, the factors that influence the fluorescence intensity change are environmental conditions, probe concentration and instrumental efficiency.15,16 Ratiometric probes can eliminate most or all vagueness by the self-calibration of the emission intensities at two wavelengths.17–21 Chen et al. designed a probe CouMC by connecting a coumarin fluorophore and an indolenium block through an ethylene group to obtain a hybrid fluorophore of coumarin and merocyanine.22 Addition of HS to interact at the merocyanine moiety in the probe CouMC altered the conjugated system and an internal charge transfer (ICT) resulting in ratiometric sensing behavior by decreasing the merocyanine emission band and increasing the coumarin emission band. Canary’s group23 reported the ratiometric displacement approach to detect Cu2+ using two fluorophore elements which demonstrated different emission at different excitation wavelengths. A great deal of work has focused on systematic studies designed to elucidate the optical properties of sensory molecules for sensing purposes. Our previous work24 based on the FRET-on process for the discrimination of catecholamines demonstrated a powerful tool for the discrimination of DA and NE from EPI using a FRET-on process induced by an intermolecular assembly of coumarin aldehyde (CA) and pyreneboronic acid (PBA) with catecholamines as a guest linker. However, this conceptual design did not show a specific detection of DA and NE. Taking the problem of discriminating between such similar structures as a particular challenge for the chemist, the discrimination of biogenic amines with similar structures prompts us to develop a more effective sensory system to solve this weakness. Considering the structures of DA and NE, NE consists of an additional hydroxyl group on the β-position of the side chain. As anticipated, this hydroxyl group close to the ammonium ion of NE might give a different binding affinity with a crown-ether moiety compared to DA without a hydroxyl group on the side chain. Taking on board the idea of intermolecular self-assembly in a spontaneously controllable manner, we have engineered a host–guest complex using two suitable sensing elements which enable covalent binding with catechol and interact with the ammonium unit based catecholamine. Apart from the interesting properties of fluorescence sensors, a dual fluorophore giving two different emission responses from the same excitation wavelength gave fascinating sensing properties.25–30 Our motivation is to design two fluorescence chemosensors: (i) NB contains boronic acid connected to a napthalimide unit for covalent binding with catechol and as a fluorophore giving the emission band at 381 nm, (ii) CC consists of a crown-ether unit connected to coumarin for a non-covalent interaction with the ammonium ion based catecholamine and as a fluorophore with an emission band at 475 nm.We hypothesized that the suitable catecholamine guest will allow the well-defined linkage between NB and CC to provide a different recognition pattern resulting in the discrimination of DA and NE as in the conceptual illustration shown in Scheme 1. It is well-known that most fluorophores were quenched by the catecholamine group due to the electron rich catechol group. The different quenching patterns of the two fluorophores upon binding with an analyte would give a different ratiometric fluorescence according to the different binding affinities. This approach can possibly discriminate catecholamine neurotransmitters in biological systems. Furthermore, PCA analysis is utilized for the classification of biogenic amine analytes using fluorescence spectral data. Additionally, we also apply this conceptual sensing in human urine samples.
Experimental
General
Nuclear magnetic resonance (NMR) spectra were recorded on Varian Mercury 400 MHz and Bruker 500 MHz nuclear resonance spectrometers (in CDCl3, DMSO-d6 and D2O). ESI HRMS spectra were recorded on a Varian Cary Eclipse spectrofluorometer.All materials and solvents were purchased from Aldrich, Fluka, Merck and TCI as standard analytical grades and used without further purification. Commercial grade solvents such as acetone, dichloromethane, methanol and ethanol were purified by distillation before using. Thin-layer chromatography (TLC) was performed on silica and alumina gel plates (Kieselgel 60 F254, 1 mm, Merck). AR grade dimethyl sulfoxide used in the fluorescence measurement was used without further purification. The synthesis of N-(1,8-naphthaloyl)-3-aminophenylboronic acid (NB) was carried out according to the reported procedure.31
Synthesis of sensor CC
2-Hydroxymethyl-18-crown-6 (0.151 g, 0.515 mmol) and triethylamine (0.36 mL, 2.575 mmol) in benzene (15 mL) were stirred at room temperature for 30 min under a nitrogen atmosphere. Then, the reaction mixture was added to a solution of coumarin acid chloride (2) in benzene (5 mL) using a cannula and refluxed for 24 hours under a nitrogen atmosphere. The solvent was removed under reduced pressure. The crude product was purified by column chromatography (Al2O3) with 10% EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 as the eluent to obtain sensor CC as a brown oil (0.171 g, 62%). 1H-NMR CDCl3 δ 8.35 (s, 1H, ArH), 7.29 (d, J = 8.8 Hz, 1H, ArH), 6.54 (dd, J = 3.47 Hz, 1H, ArH), 6.36 (d, J = 1.6 Hz, 1H, CCH), 4.39–4.24 (m, 2H, OCH2), 3.61–3.35 (m, 22H, OCH2), 3.38 (q, J = 7.07 Hz, 4H, CH2CH3), 1.19 (t, J = 7.1 Hz, 6H, CH2CH3). Elemental analysis: calcd for C27H39NO10C, 60.32; H, 7.31; N, 2.61, found: C, 60.21; H, 7.47; N, 2.42.
CH2Cl2 as the eluent to obtain sensor CC as a brown oil (0.171 g, 62%). 1H-NMR CDCl3 δ 8.35 (s, 1H, ArH), 7.29 (d, J = 8.8 Hz, 1H, ArH), 6.54 (dd, J = 3.47 Hz, 1H, ArH), 6.36 (d, J = 1.6 Hz, 1H, CCH), 4.39–4.24 (m, 2H, OCH2), 3.61–3.35 (m, 22H, OCH2), 3.38 (q, J = 7.07 Hz, 4H, CH2CH3), 1.19 (t, J = 7.1 Hz, 6H, CH2CH3). Elemental analysis: calcd for C27H39NO10C, 60.32; H, 7.31; N, 2.61, found: C, 60.21; H, 7.47; N, 2.42.
Complexation studies using fluorescence spectrophotometry
Typically, a stock solution of 1 × 10−4 M sensor NB in DMSO was prepared. All the biogenic amines (DA, NE, EPI, TY, Glu, Lys, His and Hist) were prepared at a concentration of 2 × 10−3 M in 0.01 M phosphate buffer, pH 7.4. Fluorescence spectra were recorded from 350–800 nm at ambient temperature. For fluorescence titrations, the solution of the guests was added directly to 2.00 mL of 1 × 10−5 M sensor NB in a 1 cm quartz cuvette using a micropipette and the portion of the mixture solution was stirred for 5 min prior to the measurement.Principle component analysis (PCA) method
For PCA analysis, the solution of guests (100 equiv.) was added to the solution of sensor NB (1 × 10−5 M) and stirred for 5 min. Then, 0.1 mL of 15-crown-5 was added to the solution mixture and stirred for 10 min. The solution of sensor CC (5 × 10−5 M) was added to the same 1 cm quartz cuvette using a micropipette and stirred for 5 min. The fluorescence spectra were monitored after each addition. The complexation was repeatedly detected 5 times for each guest. The evaluation of the PCA was calculated from the responsive fluorescence spectra in the range of 350 to 800 nm using MATLAB 7.11 (version R2011a).Complexation study of sensor NB with EPI in human urine samples
A stock solution of 1 × 10−4 M sensor NB was prepared in DMSO. The stock solution of 2 × 10−3 M EPI analyte was prepared in 0.01 M phosphate buffer pH 7.4 and synthetic urine was prepared in milli-Q water as listed in Table S1 in the ESI.† For the calibration curve, the synthetic urine was spiked into the 1 × 10−5 M sensor NB solution using a 100-fold diluted solution of urine. The EPI solution in various amounts was added to the mixture solution and stirred for 5 min. The fluorescence spectra were recorded from 350–800 nm at an excitation of 340 nm. Therefore, a calibration curve was built from the plot of I–I0 at the emission wavelength of 490 nm versus the concentration of EPI.Then, the 100-fold diluted solution of urine samples was spiked into the 1 × 10−5 M sensor NB solution. 40 μM of EPI solution was added to the mixture solution and stirred for 5 min. This manipulation was repeated three times. The fluorescence spectra were recorded from 350–800 nm at the excitation of 340 nm. After that, the amount found and the percent recovery were calculated from the calibration curve.
Results and discussion
Molecular sensors were designed and synthesized for the sensitive and selective detection of catecholamines through dual fluorescence responses at one excitation wavelength. The structure of catecholamines consists of two functional groups, catechol and an amino group. Therefore, the designed sensor should contain binding sites for the selective binding with each part of the catecholamine guests.Synthesis of sensor CC
The sensor NB was prepared in a single-step reaction by a nucleophilic substitution reaction between 3-aminophenylboronic acid hemisulfate and 1,8-naphthalene dicarboxylic acid anhydride in 56% yield. Sensor CC was obtained by a coupling reaction of 2 and 2-hydroxymethyl-18-crown-6 by nucleophilic substitution using triethylamine as a base (as shown in Scheme 2). In the NMR spectrum of CC, a singlet proton of coumarin showed an upfield shift from 8.68 to 8.35 ppm and other aromatic protons presented an upfield shift possibly caused by the electron donating nature of the methylene bridge as shown in Fig. S8 in the ESI.† In addition, the 13C-NMR spectrum showed ester group signals at 163.97 and 158.54 ppm. The HR-ESI mass spectrum showed an intense peak of m/z at 560.251 corresponding to the structure of CC. (Fig. S10 in the ESI†).Complexation studies of sensor NB with various guests using fluorescence spectrophotometry
The sensor NB contains a boronic acid moiety as a binding unit for the condensation reaction with the catechol group of the analyte and a napthalimide moiety as a fluorophore. The selectivity of sensor NB was examined in a biological system (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v of DMSO
9 v/v of DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphate buffer at 0.01 M, pH 7.4) toward 100 equiv. of various biogenic amines including dopamine (DA), norepinephrine (NE), epinephrine (EPI), tyramine (TY), L-glutamic acid (Glu), L-lysine (Lys), histidine (His) and histamine (Hist). The chemosensor NB showed a characteristic emission band at 384 nm. Similar to previous reports, most of the chemosensors upon complexing with catecholamine underwent the PET process since electron transfer from the phenyl based catecholamine to the napthalimide moiety causes fluorescence quenching. Likewise, sensor NB in the presence of DA and NE showed fluorescence quenching. On the contrary, sensor NB covalently bound with EPI surprisingly exhibits a strong fluorescence with a concomitant large red shift from 384 nm to 490 nm as shown in Fig. 1. This finding suggests that after complexation, the combination of the donating ability of the secondary amine based EPI and the poor acceptability of the boronic ester in the NB–EPI adduct greatly enhances the total orientation of the dipole moment to the acceptor part of the napthalimide resulting in the predominant ICT (Intramolecular Charge Transfer) state as shown in Fig. 1. In the case of DA and NE, the PET process is more dominant than the ICT process because the complex of sensor NB with DA and NE has only a slight influence on their dipole moments. On the other hand, other analytes without the catechol group including TY, Glu, Lys, His and Hist could not induce fluorescence changes implying that the boronic ester did not form in sensor NB.
phosphate buffer at 0.01 M, pH 7.4) toward 100 equiv. of various biogenic amines including dopamine (DA), norepinephrine (NE), epinephrine (EPI), tyramine (TY), L-glutamic acid (Glu), L-lysine (Lys), histidine (His) and histamine (Hist). The chemosensor NB showed a characteristic emission band at 384 nm. Similar to previous reports, most of the chemosensors upon complexing with catecholamine underwent the PET process since electron transfer from the phenyl based catecholamine to the napthalimide moiety causes fluorescence quenching. Likewise, sensor NB in the presence of DA and NE showed fluorescence quenching. On the contrary, sensor NB covalently bound with EPI surprisingly exhibits a strong fluorescence with a concomitant large red shift from 384 nm to 490 nm as shown in Fig. 1. This finding suggests that after complexation, the combination of the donating ability of the secondary amine based EPI and the poor acceptability of the boronic ester in the NB–EPI adduct greatly enhances the total orientation of the dipole moment to the acceptor part of the napthalimide resulting in the predominant ICT (Intramolecular Charge Transfer) state as shown in Fig. 1. In the case of DA and NE, the PET process is more dominant than the ICT process because the complex of sensor NB with DA and NE has only a slight influence on their dipole moments. On the other hand, other analytes without the catechol group including TY, Glu, Lys, His and Hist could not induce fluorescence changes implying that the boronic ester did not form in sensor NB.
The fluorescence changes of sensor NB in the presence of catecholamines (DA, NE and EPI) are indicative of the selective binding between sensor NB and catecholamines. To evaluate the binding mode between sensor NB and catecholamines, the stoichiometries of the complexes were measured using the Job’s method using a fluorescence technique showing the stoichiometry of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode for sensor NB with DA, NE and EPI (as shown in Fig. S12 in the ESI†).
1 binding mode for sensor NB with DA, NE and EPI (as shown in Fig. S12 in the ESI†).
Binding properties of sensor NB with DA, NE and EPI using a fluorescence spectrophotometric titration technique
To verify the binding affinity of sensor NB, fluorescence titration of sensor NB was carried out in phosphate buffer solution at pH 7.4.The fluorescence intensity at 384 nm of the complexation of sensor NB with DA, NE and EPI gradually decreased upon the increment of catecholamine guests as illustrated by Fig. 2(A)–(C). Interestingly, the new emission band at 490 nm for the complex NB–EPI was significantly developed. Fluorescence titration data were analyzed using non-linear regression plots (Fig. S13†) to provide the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks values for complex NB with DA, NE and EPI of 4.13, 4.17 and 4.01, respectively. All guests employed the catechol group to covalently bind with the boronic acid based NB. It can be reasonably explained that the ammonium ion on DA and NE preferred to promote the condensation reaction of the boronic acid and catechol group producing a negative boronate ester. It is reliably rationalized that the log
Ks values for complex NB with DA, NE and EPI of 4.13, 4.17 and 4.01, respectively. All guests employed the catechol group to covalently bind with the boronic acid based NB. It can be reasonably explained that the ammonium ion on DA and NE preferred to promote the condensation reaction of the boronic acid and catechol group producing a negative boronate ester. It is reliably rationalized that the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ks values of sensor NB with DA and NE are slightly higher than those for the sensor NB with EPI.
Ks values of sensor NB with DA and NE are slightly higher than those for the sensor NB with EPI.
It is particularly important to extensively investigate the visual detection of EPI observed from a large fluorescence change and strong fluorescence intensity. To verify the selective detection of NB towards various analytes by naked-eye sensing, the solution of NB in the presence of various analytes was exposed to 356 nm UV-visible light. The fluorescence color changes of sensor NB toward analytes are displayed in Fig. 3. The solution of sensor NB in the presence of DA or NE showed a very low brightness of luminescence. On the other hand, the solution of the NB–EPI adduct exhibited a highly green luminescence. Among other guests including TY, Glu, Lys, His and Hist, the sensor NB solution still remained unchanged.
Furthermore, to develop the EPI sensor on the solid support, filter paper was dipped in 5 × 10−5 M sensor NB solution. Then, this filter paper was painted with the word “EPI” using a paintbrush dipped in EPI solution. The prepared filter paper was exposed to 356 nm UV light, and showed a strong green luminescence of the word “EPI” as illustrated in Fig. 3(B). As a result of the fluorescence changes on the solid support, sensor NB offers an excellent detection of EPI on the paper base. The results also implied that the sensor NB can bind with EPI yielding the NB–EPI adduct on the solid support.
Determination of the detection limit of sensor NB with DA, NE and EPI using fluorescence spectrophotometry
The detection limits of sensor NB toward DA, NE and EPI measured in a range of 11.9–95.2 μM, 13.0–130.0 μM and 5.0–95.0 μM were 7.71, 8.50 and 1.54 μM, respectively. As consistent with a large fluorescence change in the NB–EPI complex, sensor NB can serve as an effective EPI sensor in a very low concentration compared to DA and NE. (Fig. S15 in the ESI†).Complexation studies of sensors NB and CC with various guests using fluorescence spectrophotometry techniques
Although sensor NB offers considerable promise as an EPI selective fluorescence probe, our further purpose is to discriminate the similar structures of DA and NE. Therefore, we developed ratiometric fluorescence probes using two fluorescence sensory elements (NB and CC) containing different binding sites and employed an intermolecular self-assembled complex to give different emission bands at the same excitation wavelength. We hypothesized that the adaptive self-assembled complexation of boronic acid based NB and crown-ether based CC by an induced fit of a proper guest linker, DA or NE, would provide different recognition patterns as a consequence of the different fluorescence responses of the dual fluorophores. Notably, naphthalimide fluorophores containing boronic acid and crown-ether coumarin exhibit different emission bands at 384 and 475 nm, respectively, upon excitation at 340 nm. Since the structures of DA and NE are different in the side chain, we expect that a different ratio of dual fluorescence responses after complexation could be obtained.The sodium cation in the phosphate buffer solution could interfere with the recognition affinity of the ammonium ion of the guest with the crown-ether based CC. To overcome this problem, a competitive receptor for Na+ ions was utilized. From the previous report, the binding constant of 15-crown-5 with Na+ ions is much higher than that of 15-crown-5 with ammonium ions.32 In all cases for the complexation studies of CC systems in buffer solution, 15-crown-5 will be added to preferentially bind with Na+ ions.
To verify the selectivity of the dual sensory system, the complexation of sensors NB and CC toward 100 equiv. of various guests including DA, NE, EPI, TY, Glu, Lys, His and Hist was evaluated in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v DMSO
9 v/v DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phosphate buffer at 0.01 M, pH 7.4. In Fig. 4(a), the observation of the fluorescence changes of mixed sensors NB and CC upon adding DA, NE, and EPI signified a very specific recognition of the dual sensory system towards catecholamines. Other biogenic amines with the two sensory system showed a small change in both emission bands indicating a non-fitted complexation for both sensing elements.
phosphate buffer at 0.01 M, pH 7.4. In Fig. 4(a), the observation of the fluorescence changes of mixed sensors NB and CC upon adding DA, NE, and EPI signified a very specific recognition of the dual sensory system towards catecholamines. Other biogenic amines with the two sensory system showed a small change in both emission bands indicating a non-fitted complexation for both sensing elements.
Upon the addition of DA or NE, the fluorescence intensity of both sensors was quenched possibly due to a PET process from the phenyl donor group to the napthalimide and coumarin acceptor groups as shown in Fig. 4. Compared to the emission band at 475 nm for sensor CC with various analytes, the emission band at 384 nm for complex NB with DA along with NE showed a larger quenching while other guests did not effect the spectral change. This suggested that napthalimide showed a stronger effect for the energy transfer from guests than coumarin in the case of DA and NE. The emission band at 475 nm for CC with NE exhibited a larger decrease (I0–I = 100 a.u.) than that with DA (shown in green and red lines, respectively, in Fig. 4(a)). It should be noted that the spectra of sensor CC with guests showed a large quenching only with NE (I0–I = 40 a.u.) and no change of emission band for CC and DA (shown in green and red lines, respectively, in Fig. 4(b)). This implies that NE could bind more strongly with sensors NB and CC than DA because the hydroxyl group of NE may undergo complementary hydrogen bonding interactions with the crown-ether based CC. For a better understanding of the binding behaviors, structures of the adduct of NB⊂NE⊂CC have been calculated using density function theory (DFT) at the B3LYP/6-31+G(d) level. Fig. 5 shows the complementary hydrogen bonding (HB1 and HB2) between the OH based NE and the oxygen based napthalimide with the hydrogen based crown-coumarin, and electrostatic force of –NH3+ and the negative charge of boronate ester. This evidence clearly supports a strong binding of the mixed sensors NB and CC with NE resulting in a larger fluorescence quenching at 475 nm compared to a single sensing element of CC. Definitely, the mixing of sensors NB and CC enables the enhancement of the binding affinity of CC with DA and NE.
Owing to a well-classified determination, this approach offers very promising features for the discriminate sensing of DA and NE. The results indicate that DA and NE act as proper guest linkers between NB and CC.
As an advantage of the different fluorescence responses upon complexation, the self-coordination between sensors NB and CC with the bridging analytes of DA or NE induced different ratiometric fluorescence intensities of coumarin and napthalimide (Icoumarin/Inapthalimide) as shown in Fig. 4(a) (inset). As a result, the ratio of the dual emission of complex NB–DA–CC of 4.84 was slightly larger than that observed for complex NB–NE–CC (R = 4.28). Fantastically, the ratiometric fluorescence changes of each complex still allowed a constant ratio with different concentrations of sensors and guests as well as different conditions in the presence of excess amounts of guests.
Principle component analysis (PCA) method for the analysis of the complexation33
Furthermore, to easily identify various biogenic amines using different recognition patterns in terms of the different fluorescence responses upon complexation, the PCA method was reliably utilized to reduce the dimensionality of the data set for easier interpretation.Due to the highly selective binding affinity of sensor NB with EPI as previously mentioned, we have examined the clustering discrimination of other biogenic amines excluding EPI.
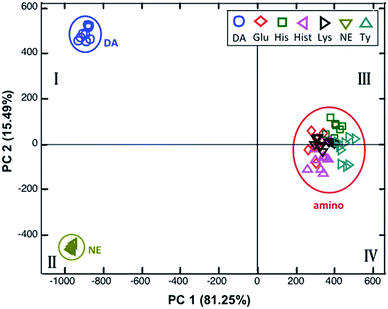
The two dimensional score plot for the two principal components (PC1 and PC2) represents 94.70% variance in the classification of biogenic amines as shown in Fig. 6. The appearance of catecholamine clusters including DA and NE on the border line between quadrant I and quadrant III was separated from other biogenic amines while the cluster of Hist and TY appeared in quadrant II. This implied that the sensor NB can bind slightly with Hist and TY. Cross-validation LDA of this model showed 77.5% accuracy in the discrimination of 7 biogenic amines.
In our further aims to separate the similar structures of DA and NE, the fluorescence spectra of mixing sensors NB and CC with biogenic amines were classified using PCA analysis. The two dimensional score plot for PC1 and PC2 representing 96.81% variance is illustrated in Fig. 6(b). This PCA model demonstrated that two sensing elements of sensors NB and CC could clearly differentiate the similar molecules of DA and NE in quadrant I and quadrant II, respectively. Hence, both sensors give a high performance for the discrimination of DA and NE while the Hist cluster appeared in quadrant III close to the cluster of sensors and other biogenic amines. Moreover, cross-validation LDA exhibited 77.5% accuracy in the discrimination of all 7 biogenic amines. The PCA score plot of the two sensing elements of NB and CC definitely gave a higher discrimination of DA and NE than the single sensing element of NB.
Complexation study of sensor NB with EPI in human urine samples
To develop the fluorescence sensor NB in analytical applications, we applied the detection of EPI to real biological systems of human urine samples. A linear range of 10–70 μM was obtained by plotting the intensity of fluorescence at 490 nm versus the spiked concentrations of EPI in the synthetic urine as indicated in Fig. S19 in the ESI.† The spiked urine samples were prepared using the 100-fold diluted solution of urine by the standard addition method. The % recovery for EPI at 40 μM of the spiked solution is in the range of 100.14–101.06% which is an acceptable recovery in sensing applications as displayed in Table 1. Therefore, the fluorescence sensor NB can serve as an effective sensor of EPI in analytical applications for real samples.Furthermore, the discrimination of catecholamine in urine samples was carried out using the PCA method as shown in Fig. 7. The two dimensional score plot for the two principal components (PC1 and PC2) represents 96.74% variance in the classification of biogenic amines. The clusters of DA and NE appeared in quadrant I and quadrant II, respectively, which were remarkably separated from other biogenic amines. This PCA plot of urine samples is consistent with that in Fig. 6. It confirmed that this dual emission system of mixed sensors NB and CC highlights an effective discrimination of DA and NE in clinical diagnosis.
 | ||
| Fig. 7 PCA score plots of mixed NB and CC upon addition of various guests (100 equiv.) in urine samples and excluding the fluorescence data of EPI for PCA analysis. | ||
Many techniques have been devoted to the determination of DA shown in Table S2.† These methods provide ultrahigh sensitivity. It can be seen from Table S2† that most of the detection limits obtained by fluorescence, electrochemistry and colorimetric methods are lower than the micromolar range. Although the detection limit of our sensory system is not better than that found in the previous reports, the normal amount of DA in human serum should be in the range of 10−7 to 10−5 M.34 The outstanding results of our sensory system are an effective discrimination of DA and NE which is definitely remarkable and fantastic and is a rare report focusing on this important approach due to quite similar structures of the guests. This aspect is still a challenge for chemists.
Conclusions
An effective discrimination of EPI, DA and NE has been achieved by the development of the ratiometric fluorescence of two sensing elements of NB and CC. EPI was clearly discriminate from other biogenic amines with a strong emission band at 490 nm. Ratiometric fluorescence sensors NB and CC displayed the emission ratio (I490/I384) for NB–DA–CC and NB–NE–CC complexes of 4.84 and 4.28, respectively, with excess amounts of guests. We have also successfully reported the obviously different recognition pattern of the two sensors NB and CC toward catecholamines using the PCA method. For analytical applications, chemosensor NB offers a good result for the detection of EPI in human urine samples. This is the first example of the significant discrimination of DA and NE by the intermolecular self-assembled recognition of two sensors. This research highlights the promising concept of intermolecular ratiometric fluorescence sensors using two sensing elements.Acknowledgements
We gratefully acknowledge the Thailand Research Fund (TRF), Commission on Higher Education (CHE) (RSA5680015 and RTA5300083), the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University (RES560530126-AM) and the 90th Anniversary of Chulalongkorn Fund (Ratchadaphiseksomphot Endowment Fund) for research grants.Notes and references
- I. Paris, A. Dagnino-Subiabre, K. Marcelain, L. B. Bennett, P. Caviedes, R. Caviedes, C. O. Azar and J. Segura-Aguilar, J. Neurochem., 2001, 77, 519–529 CrossRef CAS.
- A. Sawa and S. H. Snyder, Science, 2002, 296, 692–695 CrossRef CAS PubMed.
- A. Kar, Principles of medicinal chemistry, New Age International (P) Ltd, New Delhi, 3rd edn, 2005 Search PubMed.
- J. W. Simpkins, N. Bodor and A. Enz, J. Pharm. Sci., 1985, 74, 1033–1036 CrossRef CAS PubMed.
- E. L. Bravo, R. C. Tarazi, R. W. Gifford and B. H. Stewart, N. Engl. J. Med., 1979, 301, 682–686 CrossRef CAS PubMed.
- G. A. Kaltsas, G. M. Besser and A. B. Grossman, Endocr. Rev., 2004, 25, 458–511 CrossRef CAS PubMed.
- K. E. Secor and T. E. Glass, Org. Lett., 2004, 6, 3727–3730 CrossRef CAS PubMed.
- Z. Wu, M. Li, H. Fang and B. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 7179–7182 CrossRef CAS PubMed.
- Y. J. Jang, J. H. Jun, K. M. K. Swamy, K. Nakamura, H. S. Koh, Y. J. Yoon and J. Yoon, Bull. Korean Chem. Soc., 2005, 26, 2041–2043 CrossRef CAS.
- A. Coskun and E. U. Akkaya, Org. Lett., 2004, 6, 3107–3109 CrossRef CAS PubMed.
- F. M. Raymo and M. A. Cejas, Org. Lett., 2002, 4, 3183–3185 CrossRef CAS PubMed.
- Y. Tao, Y. Lin, J. Ren and X. Qu, Biosens. Bioelectron., 2013, 42, 41–46 CrossRef CAS PubMed.
- C.-H. Liu, C.-J. Yu and W.-L. Tseng, Anal. Chim. Acta, 2012, 745143–745148 Search PubMed.
- (a) V. S.-Y. Lin, C.-Y. Lai, J. Huang, S.-A. Song and S. Xu, J. Am. Chem. Soc., 2001, 123, 11510–11511 CrossRef CAS; (b) D. R. Radu, C.-Y. Lai, J. W. Wiench, M. Pruski and V. S.-Y. Lin, J. Am. Chem. Soc., 2004, 126, 1640–1641 CrossRef CAS PubMed.
- R. Y. Tsien and M. Poenie, Trends Biochem. Sci., 1986, 11, 450–455 CrossRef CAS.
- G. He, X. Zhang, C. He, X. Zhao and C. Duan, Tetrahedron, 2010, 66, 9762–9768 CrossRef CAS PubMed.
- R. Guliyev, A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2009, 131, 9007–9013 CrossRef CAS PubMed.
- S. Y. Lee, H. J. Kim, J. S. Wu, K. No and J. S. Kim, Tetrahedron Lett., 2008, 49, 6141–6144 CrossRef CAS PubMed.
- Z. Xu, X. Qian and J. Cui, Org. Lett., 2005, 7, 3029–3032 CrossRef CAS PubMed.
- Z. Guo, W. Zhu, L. Shen and H. Tian, Angew. Chem., Int. Ed., 2007, 46, 5549–5553 CrossRef CAS PubMed.
- H. Yu, M. Fu and Y. Xiao, Phys. Chem. Chem. Phys., 2010, 12, 7386–7391 RSC.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688–1691 CrossRef CAS PubMed.
- M. Royzen, Z. Dai and J. W. Canary, J. Am. Chem. Soc., 2005, 127, 1612–1613 CrossRef CAS PubMed.
- A. Chaicham, S. Sahasithiwat, T. Tuntulani and B. Tomapatanaget, Chem. Commun., 2013, 49, 9287–9289 RSC.
- S. R. Adams, A. T. Harootunian, Y. J. Buechler, S. S. Taylor and R. Y. Tsien, Nature, 1991, 349, 694–697 CrossRef CAS PubMed.
- M. H. Lee, D. T. Quang, H. S. Jung, J. Yoon, C.-H. Lee and J. S. Kim, J. Org. Chem., 2007, 72, 4242–4245 CrossRef CAS PubMed.
- X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025–8029 CrossRef CAS PubMed.
- Z. Zhou, M. Yu, H. Yang, K. Huang, F. Li, T. Yi and C. Huang, Chem. Commun., 2008, 3387–3389 RSC.
- V. S. Jisha, A. J. Thomas and D. Ramaiah, J. Org. Chem., 2009, 74, 6667–6673 CrossRef CAS PubMed.
- K. R. Lee and I.-J. Kang, Ultramicroscopy, 2009, 109, 894–898 CrossRef CAS PubMed.
- H. Cao, T. McGill and M. D. Heagy, J. Org. Chem., 2004, 69, 2959–2966 CrossRef CAS PubMed.
- Z. Wang, S. H. Chang and T. J. Kang, Spectrochim. Acta, Part A, 2008, 70, 313–317 CrossRef PubMed.
- R. G. Brereton, Chemometrics for Pattern Recognition, Wiley, West Sussex, United Kingdom, 2009 Search PubMed.
- J. de Champlain, L. Farley, D. Cousineau and M. R. van Ameringen, Circ. Res., 1976, 38, 109–114 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: This article contains synthesis, NMR spectra, mass spectra, fluorescence spectra and Job’s plot analysis. See DOI: 10.1039/c5ra10321e |
| This journal is © The Royal Society of Chemistry 2015 |