Successive optimisation of waste cooking oil transesterification in a continuous microwave assisted reactor†
M. A. Mohd. Alia,
R. M. Yunusa,
C. K. Chengb and
J. Gimbun*b
aFaculty of Chemical & Natural Resources Engineering, Universiti Malaysia Pahang, 26300 Gambang, Pahang, Malaysia
bCentre of Excellence for Advanced Research in Fluid Flow (CARIFF), Universiti Malaysia Pahang, 26300 Gambang, Pahang, Malaysia. E-mail: jolius@ump.edu.my
First published on 7th September 2015
Abstract
This paper presents an optimization study of waste cooking oil (WCO) transesterification in a continuous microwave assisted reactor (CMAR). The custom-built CMAR employed an integrated proportional-integral-derivative controller for accurate control of temperature and reactant flowrate. The fatty acid methyl ester contents in the sample were determined using gas chromatography mass spectrometry (GC-MS). The results from two-level factorial design showed that the methanol to oil molar ratio, amount of NaOCH3 catalyst and reaction time influenced markedly the biodiesel conversion, with the significance of 45.99%, 6.76% and 3.21%, respectively. Further analysis using a successive optimization method generated by the Box–Behnken design predicted an optimum biodiesel conversion of circa 97.13% at 0.68 wt% of catalyst loading, 11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of methanol to oil molar ratio and 4.47 min of reaction time. Experimental validation of the optimum conditions showed an excellent agreement, with a minimum deviation of 0.18% from three replicates. The biodiesel produced in this work also met the specification of ASTM D6751.
1 of methanol to oil molar ratio and 4.47 min of reaction time. Experimental validation of the optimum conditions showed an excellent agreement, with a minimum deviation of 0.18% from three replicates. The biodiesel produced in this work also met the specification of ASTM D6751.
1. Introduction
Diesel is vital energy resource in transportation, agricultural and industrial sectors. Malaysia imports about 10 million tonnes of petroleum diesel fuel costing about USD380 million annually to fulfil its domestic demand. Since early 2010, the Malaysian Government has mandated the use of B5 biodiesel to reduce the dependency on petrodiesel and hence reduce the cost associated with import of petroleum diesel. Biodiesel must be technically feasible, economically competitive, environmentally friendly, and readily available to meet the demand before it can become a viable alternative to petroleum diesel fuels.1The transesterification method using oil or fat with an alcohol under presence of an appropriate catalyst is often used to produce biodiesel. There are several different sources of oils such as palm oil, coconut oil, soybean oil, jatropha oil, rubber seed oil and waste cooking oil, which are suitable for biodiesel production. Gimbun et al.2 reported that, biodiesel using edible oil is not feasible at present due to high feedstock costs, besides also being consumed as food, which has resulted in a food vs. fuel debate. Another notable option such as using jatropha seed is seen as indirectly contributing to the food vs. fuel issue because the arable land mean for food crop is used for biofuel crop instead. Alternatively, biodiesel can be produced from lower grade oil such as waste cooking oil (WCO). The feedstock cost account for about 80% of the total production cost of biodiesel,3 thus the use of WCO can reduce the production cost markedly. Malaysians consume about 3 billion liters of cooking oil annually, from which 900 million litres of WCO are produced. The reuse of WCO for food preparation is ill-advised as it is potentially harmful to human health. Thus, WCO is a suitable feedstock for biodiesel production.
Biodiesel is often synthesized in a conventional reactor, which suffers from heat and mass transfer limitations,4 hence has lower conversion of WCO methyl esters and longer reaction time (up to 120 min) in comparison with a microwave reactor (<10 min).5 The oil feedstock account for 80% of the cost of biodiesel production. Biodiesel production using fresh oil feedstock can be costly, whereby the feedstock account for over 80% of the total cost in biodiesel production.6 Microwave reactor is known to overcome most of the aforementioned limitation and hence in recent years, the microwave irradiation heating system has been used in the transesterification process.7 The microwave-assisted transesterification offers a very short reaction time, high conversion of oil to biodiesel and the least amount of catalyst required compared to the conventional process. This is attributed to the direct energy transfer to the reactants by the microwave radiation that eliminates the preheating step.8 Many works concerning batch9–13 and continuous14–16 microwave-assisted transesterification have been reported in the literature. Continuous reactor is desired because it is easily scalable for industrial application.
Earlier design of continuous microwave reactor employed a glass reaction vessel.14 However, large vessel has a microwave penetration issue. Moreover, glass reactor is less durable when the reactor is pressurised, which often happen as a result of pumping. Other material such as poly-tetrafluoroethylene (Teflon) can withstand temperature up to 180 °C and high pressure, thus a good choice to build the microwave reactor. It is also important to have an accurate control for temperature and flow rate in the microwave reactor. The CMAR developed in this work has all the desired control features, i.e., temperature, flow rate and microwave power to ensure a precise control of the process parameter for the transesterification process. Liao and Chung15 also studied an optimisation of continuous microwave assisted transesterification of jatropha oil using a response surface methodology (RSM). However, they did not perform a successive optimisation from one parameter at time (OFAT), followed by two-level factorial (2LF) prior to the RSM study; hence the optimum condition reported in their work may be subject for further optimisation. In addition, Liao and Chung15 experimental setup did not have the control feature.
Therefore, this work focused on the optimization of continuous transesterification of waste cooking oil in a continuous microwave assisted reactor (CMAR). The effects of various variables such as catalyst loading, methanol to oil molar ratio, reaction time, temperature and microwave irradiation power on the WCO conversion and biodiesel yield was studied. These variables were screened using two-level factorial model and the response surface methodology to find the optimum condition for the WCO to biodiesel conversion in CMAR.
2. Experimental
2.1. Chemicals and oil feedstock
The analytical grade methanol (97.0%) and ethanol (99.9%) were purchased from Merck (Darmstadt, Germany). Except methanol and ethanol, all other chemicals (sodium methoxide (NaOCH3), potassium hydroxide (KOH), fuller earth, florisil and chromatography grade n-hexane) and standards (methyl laurate, methyl myristate, methyl palmitate, methyl palmitoleate, methyl stearate, methyl oleate, methyl linoleate and standard methyl heptadecanoate) were obtained from Sigma-Aldrich (St. Louis, MO).The palm oil based WCO was obtained from Sri Melekek restaurant, Malacca, Malaysia. About 80 litres of waste cooking oil was collected in a large jerry can for over a month period, and the same oil was used throughout this work to ensure a consistent feedstock. The WCO was found to have separated into two distinct layers; the upper layer was much darker and more viscous than the bottom layer which may be attributed to water contamination. Therefore, only the upper layer was used in the experiments. This upper layer was filtered through a 200 μm sieve before use. The chemical and physical properties of the oil were determined using ASTM D6751 method.
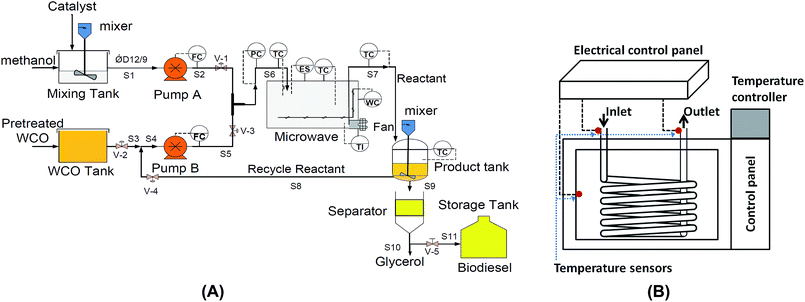
2.2. Continuous microwave assisted reactor (CMAR)
The CMAR was developed according to the process control and instrumentation diagram shown in Fig. 1. The reactor system comprised of a modified LG wavedom model (MS-2384B, South Korea) microwave oven fitted with temperature control relay (Shinko, JCS-33A, Japan) and poly-tetrafluoroethylene (Teflon) tube. This CMAR in this work is comparable to that of Liao and Chung15 but with a better control system in place. The reactor was connected to three tanks for storing the reactant and product. The catalyst was mixed homogenously in the mixing tank that contained methanol, using a mechanical stirrer (Heidolph RZR 2051 control, Germany) and the feed flow rate was controlled using two solenoid metering pump (Prominent BT4b, Germany).2.3. Transesterification and biodiesel purification
Transesterification is the most commonly used method to produce biodiesel because it is a low-cost process and involved simple reaction. The transesterification process of WCO was performed using the CMAR in the presence of excess methanol to favour the yield of methyl esters. At first, the WCO was bleached with fuller earth and heated to a temperature of 80 °C for 30 min followed by centrifugation at 4629 × g (Eppendorf 5810R), to remove impurities. The oil was heated to the desired reaction temperature prior to transesterification process. A pre-determined amount of sodium methoxide (catalyst) was mixed with a settled amount of methanol using a stirrer until homogeneous blend was obtained. Both WCO and methanol (with sodium methoxide) were pumped continuously to the CMAR using two solenoid metering pumps. The conditions studied were the methanol to WCO ratio (from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 21
1 to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1), catalyst loading (from 0.75 to 1.25 wt%), reaction temperature (from 55 °C to 65 °C), microwave irradiation power (from 180 W to 900 W) and reaction time (from 4 to 7 min). The flow rate of the dosing pump was set constant at 100% stroke, which was equal to 180 strokes per minute (or 308 ml minute−1) upon calibration (R2 = 0.997). The temperature sensors were placed in the inlet and outlet of the Teflon pipe and the reaction started when the set temperatures was achieved. The reaction time is controlled by the timer installed at the electrical control panel whereby the oil re-circulation automatically stops after the pre-set reaction time is reached. In this prototype, the reactant is allowed to re-circulate inside the microwave reactor until the pre-set reaction time is reached.
1 mol mol−1), catalyst loading (from 0.75 to 1.25 wt%), reaction temperature (from 55 °C to 65 °C), microwave irradiation power (from 180 W to 900 W) and reaction time (from 4 to 7 min). The flow rate of the dosing pump was set constant at 100% stroke, which was equal to 180 strokes per minute (or 308 ml minute−1) upon calibration (R2 = 0.997). The temperature sensors were placed in the inlet and outlet of the Teflon pipe and the reaction started when the set temperatures was achieved. The reaction time is controlled by the timer installed at the electrical control panel whereby the oil re-circulation automatically stops after the pre-set reaction time is reached. In this prototype, the reactant is allowed to re-circulate inside the microwave reactor until the pre-set reaction time is reached.
The product was decanted into a separatory funnel and allowed to settle for 24 h to attain two distinct layers. The upper layer was comprised of waste cooking oil methyl esters (WCOME) whereas the bottom part was comprised of glycerol, catalyst and other impurities. The residual methanol and glycerol were then washed from WCOME using warm water (60 °C). Subsequently, florisil (MgSiO3) was added to the WCOME and stirred vigorously at 40 °C to remove water residue before being centrifuged. The WCOME was filtered through Whatman (125 μm) filter papers prior to chemical, physical and GCMS analysis according to ASTM D6751 standard.
2.4. Experimental design
The optimisation study was performed with the aid of the Design Expert software (Stat-Ease Inc., Minneapolis, US, Version 8.0.4). The range of parameter was first identified via OFAT study before the 2LF design model was used to determine the significance of each variable. The independent variables such as catalyst loading (X1), methanol to oil molar ratio (X2), reaction time (X3), temperature (X4) and microwave irradiation power (X5) in this work were chosen based on our previous OFAT studies (Mohd Ali et al.16) and the conditions studied is shown in Table 1. The variables in the experiment were developed and coded into levels, α = −1, 0 and +1. The model comprises a two-level small factorial design (½ × 25 = 16 experiments as shown in Table 2).| Properties | This work | Reference10,19 |
|---|---|---|
Palmitic acid C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
39.84 | 36.95 |
Stearic acid C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4.17 | 4.85 |
Oleic acid C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43.73 | 46.25 |
Linoleic acid C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
7.87 | 10.51 |
Methyl laurate C12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.52 | 1.2 |
Methyl myristate C14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
1.16 | — |
Methyl palmitate C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
35.76 | 36.9 |
Methyl palmitoleate C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.63 | — |
Methyl stearate C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4.60 | 6.7 |
Methyl oleate C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
41.06 | 31.6 |
Methyl linoleate C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
8.78 | 18.9 |
Methyl arachidate C20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.51 | 0.7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Chemical properties of WCO | ||
| FFA (%) | 1.14 | 1.01 |
| Iodine value (g per 100 g) | 78.38 | 86.0 |
| Saponification value (mg g−1) KOH | 202.74 | 209.0 |
| Variable | OFAT study | Two-level of factorial study | |||||
|---|---|---|---|---|---|---|---|
| Range | Maximum differences (%) | OFAT highest conversion | Sum of squares | p value prob > F | Percentage contribution (%) | 2LF highest conversion | |
| a Variables with highest contribution and p < 0.05 from 2LF.b The most significant variables from OFAT study.c R2 = 0.98, adj R2 = 0.94, F-value = 25.82. | |||||||
| Model | 117.29 | 0.0033 | Significant | ||||
| x1-catalyst loading | 0.75–1.25 | 10.16b | 1.0 | 3.81 | 0.0385a | 3.21a | 0.76 |
x2-methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12 1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
29.36b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
54.70 | 0.0003a | 45.99a | 11.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| x3-reaction time | 5–7 | 3.2b | 6 | 8.04 | 0.0116a | 6.76a | 5.24 |
| x4-temperature | 55–65 | 2.4 | 60 | 1.99 | 0.0932 | 1.67 | 60 |
| x5-microwave power | 540–900 | 3.1 | 720 | 0.45 | 0.3544 | 0.38 | 900 |
| % Conversion | 97.40 | 97.43 | |||||
The most significant effects from 2LF analysis were chosen for the response surface method to determine the optimum biodiesel conversion. The 2LF study indicated that the catalyst loading (x1), methanol to oil molar ratio (x2) and reaction time (x3) are the most significant variables to achieve higher biodiesel conversion. The chosen range for parameters x1, x2 and x3 were 0.60 to 0.90 wt%, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 13
1 to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4 to 6 min, respectively. Each variable in the experiment was developed and coded into levels −1, 0 and +1 as shown in Table 3. Box–Behnken factorial design model was used since the model is suitable for a continuous process.15 The model required 15 experiments as shown in Table 4 and were carried out in randomized order.
1 and 4 to 6 min, respectively. Each variable in the experiment was developed and coded into levels −1, 0 and +1 as shown in Table 3. Box–Behnken factorial design model was used since the model is suitable for a continuous process.15 The model required 15 experiments as shown in Table 4 and were carried out in randomized order.
| Design points | Process variables | Conversion (%) | |||
|---|---|---|---|---|---|
| x1 catalyst loading (wt%) | x2 methanol to oil ratio | x3 reaction time (min) | Experimental | Predicted | |
| 1 | 0.6(−1) | 11(−1) | 5(0) | 96.10 | 95.64 |
| 2 | 0.9(1) | 11(−1) | 5(0) | 92.72 | 92.50 |
| 3 | 0.6(−1) | 13(1) | 5(0) | 91.06 | 91.30 |
| 4 | 0.9(1) | 13(1) | 5(0) | 90.53 | 91.00 |
| 5 | 0.6(−1) | 12(0) | 4(−1) | 94.00 | 93.87 |
| 6 | 0.9(1) | 12(0) | 4(−1) | 92.00 | 91.65 |
| 7 | 0.6(−1) | 12(0) | 6(1) | 90.00 | 90.35 |
| 8 | 0.9(1) | 12(0) | 6(1) | 89.00 | 89.13 |
| 9 | 0.75(0) | 11(−1) | 4(−1) | 92.72 | 93.31 |
| 10 | 0.75(0) | 13(1) | 4(−1) | 90.32 | 90.21 |
| 11 | 0.75(0) | 11(−1) | 6(1) | 90.00 | 90.11 |
| 12 | 0.75(0) | 13(1) | 6(1) | 87.95 | 87.37 |
| 13 | 0.75(0) | 12(0) | 5(0) | 96.02 | 96.33 |
| 14 | 0.75(0) | 12(0) | 5(0) | 97.50 | 96.33 |
| 15 | 0.75(0) | 12(0) | 5(0) | 95.47 | 96.33 |
| Source | Sum of squares | Degrees of freedom (df) | Mean squares | F-value | p-value, prob > F |
|---|---|---|---|---|---|
| a R2 = 0.97, adj R2 = 0.91, C.V = 0.94%, Std. Dev = 0.86. | |||||
| Model | 113.90 | 9 | 20.66 | 16.96 | 0.0031 significant |
| A-x1 – catalyst loading | 5.97 | 1 | 5.97 | 8.00 | 0.0367 |
| B-x2 – methanol to oil | 17.05 | 1 | 17.05 | 22.86 | 0.0050 |
| C-x3 – reaction time | 18.27 | 1 | 18.27 | 24.49 | 0.0043 |
| AB (x1x2) | 2.03 | 1 | 2.03 | 2.72 | 0.1599 |
| AC (x1x3) | 0.25 | 1 | 0.25 | 0.34 | 0.5878 |
| BC (x2x3) | 0.031 | 1 | 0.031 | 0.041 | 0.8474 |
| A2 (x12) | 6.85 | 1 | 6.85 | 9.19 | 0.0290 |
| B2 (x22) | 20.65 | 1 | 20.65 | 27.68 | 0.0033 |
| C2 (x32) | 51.03 | 1 | 51.03 | 68.40 | 0.0004 |
| Residual | 3.73 | 5 | 0.75 | ||
| Lack of fit | 1.53 | 3 | 0.51 | 0.46 | 0.7384 not significant |
| Pure error | 2.20 | 2 | 1.10 | ||
| Cor total | 117.63 | 14 | |||
2.5. Statistical analysis
Data obtained from Box–Behnken design model was used to create a response surface methodology (RSM) plot and fitted into a second-order polynomial equations as shown in eqn (1). The conversion of WCO into biodiesel was chosen as a desired response, Y. The general form of the second-order equation is given by:
 | (1) |
| Y = βo + β1x1 + β2x2 + β3x3 + β12x1x2 + β13x1x3 + β23x2x3 + β11x12 + β22x22 + β33x32 | (2) |
2.6. WCO and WCOME composition analysis
Oil and FAME composition of oil was determined using gas chromatography mass spectroscopy (GC-MS) according to ASTM D6584. Initially, the sample was dissolved in HPLC grade n-hexane before injected into the GC-MS. Tri-acylglycerides (TAG) analysis was performed on Agilent 7890A gas chromatography system equipped with Agilent 7683B series injector, 5975C inert MSD and a DB-1(MS) capillary column (30 m × 0.25 mm ID × 0.25 μm films), with a temperature range of 60 to 340 °C, while the FAME produced were analyzed on HP-5 capillary column (30 m × 0.25 mm ID × 0.25 μm) with a temperature range of 60 to 325 °C. Identification of the peaks was performed by comparing the mass spectroscopy library and retention times with the standard analyzed under the same condition. The methyl esters content in the sample was quantified by comparing the peak areas percentage obtained by GC-MS. The methyl esters standard from Sigma-Aldrich was prepared in the concentration ranging from 0.6–47.2 μl ml−1 to develop a calibration curve. The data showed excellent linearity with R2 > 0.999 for methyl laurate, methyl myristate, methyl palmitate, methyl palmitoleate, methyl stearate, methyl oleate and methyl linoleate. Thus similar method was employed to provide a qualitative and quantitative analysis of biodiesel in this work. The conversion of biodiesel was estimated as follows:5,17
 | (3) |
| MWester = ∑(MWi × % mi) + 14 | (4) |
3. Results and discussion
3.1. WCOME composition and properties
Results from GC-MS analysis showed that most of the fatty acids in the WCO are made up of 12 to 24 carbon atoms with different atomic bonding, so the fatty acids displayed all double bonds in the cis and trans isomerism by one methylene group. The chain length and number of double bonds affect the physical properties of fatty acids. Therefore, a higher number of double bonds decreases the fatty acid viscosity which in turn may affect its rheological properties.18 As shown in Table 1. The WCO and WCOME compositions are comparable to the value reported in the literature.10,19 Minor differences in the WCO content is likely due to the prior-use of the oil. The WCO in this work was collected from a restaurant that cooks only small fish and chicken. Some fatty residual from the fish and chicken may be present in the WCO which accounts for the difference in oil composition. The GC-MS data shows that the methyl esters composition has methyl laurate (0.68 wt%), methyl myristate (1.34 wt%), methyl palmitate (36.99 wt%), methyl palmitoleate (2.04 wt%), methyl stearic (5.42 wt%), methyl oleic (45.26 wt%) and methyl linoleate (5.47 wt%) with 97.13 wt% of conversion. Further testing of the acid value (ASTM D664), kinematic viscosity (ASTM D445), density (ASTM D5002), flash point (ASTM D93), calorific value (ASTM D240), cloud point (ASTM D2500), pour point (ASTMD97), cetane number (ASTM D613) and moisture content (ASTM D2709) confirmed that the WCOME obtained from this work is within the range specified by ASTM6751.3.2. Effects of variables in OFAT study
The effects of five independent variables, namely the catalyst loading, methanol to oil molar ratio, reaction time, temperature and microwave irradiation power on the conversion of biodiesel was studied in our previous work.16 The range of variables for the OFAT study are shown in Table 2. Results from the OFAT experiment confirmed the suitability of the variables range studied with clearly visible peak of the highest biodiesel conversion from the plotted data for each variable (ESI†). The catalyst loading (NaOCH3) and methanol to oil molar ratio significantly affect (>10% differences) the WCO conversion to biodiesel. The effects of NaOCH3 catalyst loading (from 0.50 to 1.00 wt%) on biodiesel conversion was increased from 86.58% to 97.36%. This increment could be attributed to the increase in basic sites available for methanol to form methoxide anion. Meanwhile, the conversion decreases from 97.36% to 81.72% shown that the emulsion formed by leading to soap formation and reduced the conversion when increases further the catalyst loading from 1.00 to 1.50 wt%. The amount of methanol to oil molar ratio from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 shows, increase conversion from 64.65% to 94.01%, respectively. However, a reduction in conversion to 89.38% was observed at molar ratio, 12
1 shows, increase conversion from 64.65% to 94.01%, respectively. However, a reduction in conversion to 89.38% was observed at molar ratio, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This molar ratio shows leads to decreased conversion due to the increased methanol–glycerol solubility which interferes with the glycerol separation. Subsequent with the presence of the polar hydroxyl group in methanol promotes emulsification of the biodiesel.
1. This molar ratio shows leads to decreased conversion due to the increased methanol–glycerol solubility which interferes with the glycerol separation. Subsequent with the presence of the polar hydroxyl group in methanol promotes emulsification of the biodiesel.
Meanwhile, the effect of temperature, reaction time and microwave irradiation power were not noticeable. The reaction temperature of 50 °C was sufficient to achieve a high conversion of 95.51%. From 45 to 65 °C, >95% the biodiesel conversion was observed. At the reaction temperature of 60 °C, the highest biodiesel conversion of 97.91% was attained. A decreasing trend of conversion 95.44% and 84.64% was observed with increasing reaction temperature at 65 °C and 70 °C, respectively. This is attributed to the evaporation of methanol above 65 °C.2 Formation of bubble slug inside the Teflon tube was observed at temperature above the boiling of methanol (64.7 °C), which may inhibit the reaction. The difference in conversion to biodiesel from 50 to 65 °C was negligible (<2.4%), except for the reaction at 70 °C which is over 10% lower than the other tested temperature. This indicated that reaction temperature was not a dominant factor influencing the biodiesel conversion.
The highest conversion (97.87%) of WCOME was obtained at microwave irradiation power of 720 W, but minimal gain when the microwave irradiation power increased further up to 900 W. Limited changes in biodiesel conversion (<3.1%) was achieved by varying the microwave irradiation power from 360 to 900 W. The effects of reaction time on biodiesel conversion are also limited (<3.2%) when the time was varied from 4 to 8 min. A maximum of biodiesel conversion of 97.89% was observed at 6 min and thereafter it reduces slightly to 94.74%. This was caused by the slow reaction rate due to dispersion and mixing between the methanol and oil. The slight drop in conversion could be partly associated to the formation of glycerol under longer duration. This enhanced the hydrolysis of esters (reversed transesterification) resulting in the loss of esters as well as causing more fatty acids to form soap. The range for variable obtained from OFAT was then used for 2LF study to screen the interaction and to study the contribution of each variable on biodiesel conversion systematically.
3.3. Two-level factorial analysis
The results obtained in the 2LF experiments showed the biodiesel conversion ranged from 88.64% to 97.44%. The 2LF design model shows the most significant factor affecting biodiesel conversion are x1 (3.21%), x2 (45.99%) and x3 (6.76%). The result from 2LF study further confirmed the finding of the OFAT study which also indicates similar significant variables. The value for percentage of contribution (2LF) and percentage differences (OFAT) is not equivalent because the 2LF takes into account the statistical average of all data, whereas the percentage difference from OFAT is a merely the difference between the maximum and minimum value. Kamath et al.20 also reported a similar finding, that x1, x2 and x3 are the most significant variables affecting conversion of Karanja oil in a batch microwave irradiation reactor. The 2LF design model is given as follows:| Y% = 93.77 − 0.49x1 + 1.85x2 + 0.35x3 − 0.71x4 − 0.17x5 − 1.06x1x2 + 0.35x1x3 + 0.58x1x4 − 0.37x1x5 + 1.07x2x4 − 0.39x4x5 | (5) |
The regression shows R2 = 0.98 between the predicted versus experimental values indicating an excellent agreement (R2 value closed to unity), which mean that the data fit well with the model and can provide a good estimate of response for the system in the range studied. The eqn (5) showed the highest conversion of 97.46% can be achieved using 11.71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1 of methanol to oil molar ratio, time of 5.24 min, catalyst loading at 0.76 wt%, temperature at 60 °C and 900 W of irradiation power. Experimental validation was performed to check the validity of the model. The essentially similar conversion of 97.03 ± 0.44% was obtained from three replicate of experimental data, indicating the 2LF model is valid for the range of variable studied in this work.
1 mol mol−1 of methanol to oil molar ratio, time of 5.24 min, catalyst loading at 0.76 wt%, temperature at 60 °C and 900 W of irradiation power. Experimental validation was performed to check the validity of the model. The essentially similar conversion of 97.03 ± 0.44% was obtained from three replicate of experimental data, indicating the 2LF model is valid for the range of variable studied in this work.
3.4. Regression model development
The Box–Behnken design model was used to develop a quadratic polynomial equation. The results obtained in the experiments are sumarised in Table 3 showed the biodiesel conversion ranged from 87.95% to 97.50%. The affected variable was analysed by identifying which factors contributed to the regression model. The quadratic model was suggested as a best-fit model with p-value of 0.0011. The calculated probability value (p-value) from the analysis of the model was 0.0031 and F-value was 16.96, demonstrating a high significance of the regression model as shown in Table 4. The p- and F-values for the ‘lack of fit’ were 0.7384 (or 73.84%) and 0.46, respectively, indicating that the lack of fit was insignificant and therefore the model was considered well fit.The analysis showed that the predicted model fitted very well with the experimental data, with R2 = 0.97 between the model prediction and experiment. Moreover, adjusted R2 and coefficient of variation (CV) were 0.91 and 0.94%, which indicated that the polynomial regression model is significant and reliable.
The results in Table 4 show that interactions between the process variables may significantly affect the biodiesel conversion. The p-value (prob > F) is less than 0.05 indicated that the model terms x1, x2, x3, x12, x22 and x32 have a significant effect on the biodiesel conversion. Whereas, values greater than 0.1 indicate the model terms are not significant. That means the quadratic coefficient for x1x2, x1x3 and x2x3 is not an important factor affecting the biodiesel conversion. The independent variables, quadratic and interaction coefficient is more significant if the F-value is larger and p-value is smaller. The results showed that x2 and x3 had the greater effect on the conversion of WCOME with p-value of 0.0050 and 0.0043, respectively, compared to x1 (0.0367). The p-value for the interaction coefficient were x12 (0.0290), x22 (0.0033) and x32 (0.0004), respectively and are contributing significantly to the design model. The design equation is given as follows:
| Y% = 96.33 − 0.86x1 − 1.46x2 − 1.51x3 + 0.71x1x2 + 0.25x1x3 + 0.088x2x3 − 1.36x12 − 2.37x22 − 3.72x32 | (6) |
The design equation represent the correlation between independent variables (x1, x2 and x3) and the conversion of biodiesel. The normal plot of residual showed the point of cluster around the straight line implying that the model fits well with the data. Meanwhile, the plot of residual versus predicted response shows that the point of cluster were equally distributed a shown in the ESI.†
3.5. Effects of variable on response surface
The 3D response surface, 2D contour plot and the interaction graph was used to determine the optimum condition for transesterification of WCO using CMAR. Fig. 2 shows the interaction between the methanol to oil molar ratio versus catalyst loading at a fixed reaction time of 4.47 min. The results showed that increasing the methanol to oil molar ratio from 11.18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 11.91
1 to 11.91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and catalyst loading from 0.60 to 0.75 wt% increased the WCO conversion from 93.0% to 96.0%. Further increase of catalyst loading beyond 0.75 wt% showed no significant improvement in biodiesel conversion which may be attributed to soap formation. Moreover, soap formation complicates the separation of glycerol from biodiesel. Meanwhile, increasing methanol to oil molar ratio beyond 12
1 and catalyst loading from 0.60 to 0.75 wt% increased the WCO conversion from 93.0% to 96.0%. Further increase of catalyst loading beyond 0.75 wt% showed no significant improvement in biodiesel conversion which may be attributed to soap formation. Moreover, soap formation complicates the separation of glycerol from biodiesel. Meanwhile, increasing methanol to oil molar ratio beyond 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 produced excess methanol which increased the oil solubility in solvent causing the emulsification during product washing, thus decreased the production of biodiesel.11
1 produced excess methanol which increased the oil solubility in solvent causing the emulsification during product washing, thus decreased the production of biodiesel.11
 | ||
| Fig. 2 Effect of catalyst loading (X1) versus methanol to oil molar ratio (X2) at fixed reaction time 4.47 minute; 3D response surface plot. | ||
This work employed a successive RSM i.e. by fixing the previously optimised value of the subsequent optimisation study to obtain the highest WCO conversion. Therefore, a surface response for catalyst and reaction time was performed by fixing the previously optimised methanol to oil molar ratio of 11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 3 shows the WCO conversion increased from 92.0% to 96.0% when the reaction time increased from 4.40 to 5.20 minutes, but decreased afterwards. This is due to the reversible nature of transesterification reaction besides increases in soap formation at prolonged reaction time. Similar trends were also reported by Kamath et al.,20 who studied the optimization on Karanja oil using 1.33 wt% of KOH in a batch reactor. The WCO conversion increased when the catalyst loading was increased from 0.60 wt% before peaking at 0.73 wt%; nevertheless, increasing the catalyst loading further did not improve the WCO conversion. The response surface (97.0% conversion) peaked at catalyst loading of 0.68 wt% and the reaction time of 4.75 min. The transesterification under microwave irradiation is more efficient and less time consuming to produce biodiesel than other process. For instance, a reactor with conventional heating system took about 2 h to achieve an optimum conversion of 86.5%, using similar feedstock and catalyst.21 The reactor designed in this work also has a better performance than the comparable study by Lin et al.12 who studied WCO transesterification in the batch microwave reactor. Lin et al.12 reported about the same optimum WCO conversion (97.1%) but their catalyst loading was more than 30% higher and the reaction time was 40% longer, at 7 min.
1. Fig. 3 shows the WCO conversion increased from 92.0% to 96.0% when the reaction time increased from 4.40 to 5.20 minutes, but decreased afterwards. This is due to the reversible nature of transesterification reaction besides increases in soap formation at prolonged reaction time. Similar trends were also reported by Kamath et al.,20 who studied the optimization on Karanja oil using 1.33 wt% of KOH in a batch reactor. The WCO conversion increased when the catalyst loading was increased from 0.60 wt% before peaking at 0.73 wt%; nevertheless, increasing the catalyst loading further did not improve the WCO conversion. The response surface (97.0% conversion) peaked at catalyst loading of 0.68 wt% and the reaction time of 4.75 min. The transesterification under microwave irradiation is more efficient and less time consuming to produce biodiesel than other process. For instance, a reactor with conventional heating system took about 2 h to achieve an optimum conversion of 86.5%, using similar feedstock and catalyst.21 The reactor designed in this work also has a better performance than the comparable study by Lin et al.12 who studied WCO transesterification in the batch microwave reactor. Lin et al.12 reported about the same optimum WCO conversion (97.1%) but their catalyst loading was more than 30% higher and the reaction time was 40% longer, at 7 min.
 | ||
Fig. 3 Effect of catalyst loading (X1) versus reaction time (X3) at fixed methanol to oil molar ratio, 11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 3D response surface plot. 1; 3D response surface plot. | ||
The effect of methanol to oil molar ratio and reaction time on the WCO conversion at fixed catalyst loading (0.68 wt%) is shown in Fig. 4. The highest WCO conversion was found at reaction time of 4.47 minutes and methanol to oil molar ratio of 11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This result is comparable to the earlier study by Zhang et al.13 who reported optimum conversion yellow horn oil at methanol to oil molar ratio of 12
1. This result is comparable to the earlier study by Zhang et al.13 who reported optimum conversion yellow horn oil at methanol to oil molar ratio of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The slight difference in the methanol requirement in this work is attributed to the difference in feedstock oil used. Moreover, this work is a continuous process, while the earlier study by Zhang et al.13 is a batch process. They also employed a microwave assisted reactor, but it was a batch system instead of continuous reactor in this work. Further increase of methanol to oil molar ratio started forming emulsification and which leads to the formation of gels. The increase of molar ratio beyond 12
1. The slight difference in the methanol requirement in this work is attributed to the difference in feedstock oil used. Moreover, this work is a continuous process, while the earlier study by Zhang et al.13 is a batch process. They also employed a microwave assisted reactor, but it was a batch system instead of continuous reactor in this work. Further increase of methanol to oil molar ratio started forming emulsification and which leads to the formation of gels. The increase of molar ratio beyond 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 caused the excessive formation of glycerol, which made the separation difficult and thereby reducing the conversion of biodiesel.
1 caused the excessive formation of glycerol, which made the separation difficult and thereby reducing the conversion of biodiesel.
 | ||
| Fig. 4 Effect of methanol to oil molar ratio (X2) versus reaction time (X3) at catalyst loading, 0.68 wt%.; 3D response surface plot. | ||
3.6. Optimisation study
The methanol to oil molar ratio, catalyst loading and reaction time have significant effect on the biodiesel conversion in CMAR. This variable was set in a range between low and high levels which was coded as −1 and +1, respectively. The desired response chosen was a maximum conversion of biodiesel. The elliptical profile shown in Fig. 5 of the independent variables illustrates the position of the optimum condition and their effect on biodiesel conversion. The optimum condition for biodiesel production in CMAR was achieved at the reaction time (4.47 minutes), methanol to oil molar ratio (11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and catalyst loading (0.68 wt%) with the highest predicted conversion of 96.96%. The desirability value for optimum condition is 0.944 closed to the maximum value of 1.0. The optimum point is considered good because it is located within the range of parameters studied, closer to the middle of the 2D contour as shown in Fig. 6. The designated centre point of the model has a good predictability due to many replicates run for the Box–Behnken model in that region.
1) and catalyst loading (0.68 wt%) with the highest predicted conversion of 96.96%. The desirability value for optimum condition is 0.944 closed to the maximum value of 1.0. The optimum point is considered good because it is located within the range of parameters studied, closer to the middle of the 2D contour as shown in Fig. 6. The designated centre point of the model has a good predictability due to many replicates run for the Box–Behnken model in that region.
The optimum value predicted by the response surface model was validated experimentally to verify the accuracy of the model. Triplicate experiment for the optimum CMAR condition i.e. reaction time (4.47 minutes), methanol to oil molar ratio (11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and catalyst loading (0.68 wt%) showed a very small difference of about 0.18% between the predicted (96.96%) and actual conversion (97.13%). Therefore, the Box–Behnken design model was considered to be a valid optimization model for WCO conversion in CMAR.
1) and catalyst loading (0.68 wt%) showed a very small difference of about 0.18% between the predicted (96.96%) and actual conversion (97.13%). Therefore, the Box–Behnken design model was considered to be a valid optimization model for WCO conversion in CMAR.
Result from the successive optimisation strategy through OFAT, 2LF and RSM is summarised in Table 5. The optimised condition reduced the reaction time markedly by 25.5% (from 6 to 4.47 min) and catalyst loading by 32% (from 1 wt% to 0.68 wt%) without significantly affecting the biodiesel conversion. Nevertheless, the optimised condition requires more methanols (16.2%) than that of OFAT solution. Overall, the optimised solution is faster and requires less catalyst than that of non-optimised condition. The optimised solution is favourable if the methanol cost is cheaper and the catalyst cost is higher or the demand for production is high.
| Variables | OFAT | 2LF | RSM |
|---|---|---|---|
| Catalyst loading (wt%) | 1.0 | 0.78 | 0.68 |
| Methanol to oil (mol mol−1) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Reaction time (min) | 6 | 5.04 | 4.47 |
| Conversion (%) | 97.87 | 97.46 | 97.13 |
4. Conclusions
Transesterification of WCO using the custom-built CMAR in this work showed excellent capability to produce biodiesel effectively and efficiently. The prototype developed in this work can be further enhanced to include control and automation in the methanol–WCO pre-mixing stage as well as the possibility to use a continuous FAME separation by decanter or centrifuge. The OFAT study confirmed the suitability of the range chosen for individual variable screening. The highest biodiesel conversion obtained from OFAT study was 97.87%. The 2LF study showed that the most important variables affecting the WCO conversion were the catalyst loading, methanol to oil molar ratio and the reaction time which confirms the OFAT results. The optimum WCO conversion (97.13%) was found at 11.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol mol−1 of methanol to oil molar ratio, 4.47 minutes of reaction time and 0.68 wt% of catalyst loading using the Box–Behnken design model. Experimental validation of the response surface model showed a minimal difference (0.18%) between the predicted (96.96%) and actual conversion (97.13%). Hence, the model developed in this work is considered to be a valid optimization model for WCO conversion in CMAR. The successive optimisation techniques successfully reduce the reaction time by 25.5% and catalyst loading by 32% without significantly affecting the biodiesel conversion. The technique outlined in this work may be useful to reduce biodiesel production cost in CMAR.
1 mol mol−1 of methanol to oil molar ratio, 4.47 minutes of reaction time and 0.68 wt% of catalyst loading using the Box–Behnken design model. Experimental validation of the response surface model showed a minimal difference (0.18%) between the predicted (96.96%) and actual conversion (97.13%). Hence, the model developed in this work is considered to be a valid optimization model for WCO conversion in CMAR. The successive optimisation techniques successfully reduce the reaction time by 25.5% and catalyst loading by 32% without significantly affecting the biodiesel conversion. The technique outlined in this work may be useful to reduce biodiesel production cost in CMAR.
Conflicts of interest
The author declares no conflict of interest.Acknowledgements
We acknowledge Universiti Malaysia Pahang (GRS140304) and Ministry of Higher Education for the MTUN COE grant RDU121216 for funding this project and also YTL Pahang Cement Sdn. Bhd. for providing the limestone.Notes and references
- C. D. M. de Araújo, C. C. de Andrade, E. D. S. e. Silva and F. A. Dupas, Renewable Sustainable Energy Rev., 2013, 27, 445–452 CrossRef PubMed.
- J. Gimbun, et al., Procedia Eng., 2013, 53, 13–19 CrossRef CAS PubMed.
- A. Demirbas, Energy Policy, 2007, 35, 4661–4670 CrossRef PubMed.
- M. A. Surat, S. Jauhari and K. R. Desak, Appl. Sci. Res., 2012, 4, 645–661 Search PubMed.
- A. N. Phan and T. M. Phan, Fuel, 2008, 87, 3490–3496 CrossRef CAS PubMed.
- M. J. Haas and T. A. Foglia, Alternative feedstocks and technology for biodiesel production, in The Biodiesel Handbook, ed. G. Knothe and J. van Gerpen, AOCS Press, Urbana, IL, 2005, pp. 42–61 Search PubMed.
- F. Motasemi and F. N. Ani, Renewable Sustainable Energy Rev., 2012, 16, 4719–4733 CrossRef CAS PubMed.
- V. G. Gude, P. Patil, E. Martinez-guerra, D. Shuguang and N. Nirmalakhandan, Sustainable Chem. Processes, 2013, 1–31 Search PubMed.
- M. Koberg, R. Abu-Much and A. Gedanken, Bioresour. Technol., 2011, 102, 1073–1078 CrossRef CAS PubMed.
- Z. Yaakob, B. H. Ong, S. K. S. Kumar and M. N. Kamarudin, Int. J. Sustain. Energ., 2009, 28, 195–201 CrossRef PubMed.
- P. D. Patil, V. G. Gude, M. Aravind, P. Cooke, S. M. McGee, N. Nirmalakhandan, P. Lammers and S. Deng, Bioresour. Technol., 2011, 102, 1399–1405 CrossRef CAS PubMed.
- Y. C. Lin, P. M. Yang, S. C. Chen and J. F. Lin, Fuel Process. Technol., 2013, 115, 57–62 CrossRef CAS PubMed.
- S. Zhang, Y. G. Zu, Y. J. Fu, L. Meng, D. Y. Zhang and T. Efferth, Bioresour. Technol., 2010, 101, 931–936 CrossRef CAS PubMed.
- T. M. Barnard, N. E. Leadbeater, M. B. Boucher, L. M. Stencel and B. A. Wilhite, Energy Fuels, 2007, 21, 1777–1781 CrossRef CAS.
- C. C. Liao and T. W. Chung, Chem. Eng. Res. Des., 2011, 89, 2575–2581 CrossRef CAS PubMed.
- M. A. Mohd Ali, J. Gimbun, C. K. Cheng and R. Mohd Yunus, 8th Malaysian Technical University Conference on Engineering Technology, Melaka, Malaysia, 2014, pp. 269–273 Search PubMed.
- M. R. Shahbazi, B. Khoshandam, M. Nasiri and G. Masoud, J. Taiwan Inst. Chem. Eng., 2012, 43, 504–510 CrossRef CAS PubMed.
- A. E. Atabani, A. S. Silitonga, I. A. Badruddin, T. M. I. Mahlia, H. H. Masjuki and S. Mekhilef, Renewable Sustainable Energy Rev., 2012, 16, 2070–2093 CrossRef PubMed.
- R. Abd Rabu, I. Janajreh and D. Honnery, Energy Convers. Manage., 2013, 65, 764–769 CrossRef CAS PubMed.
- H. V. Kamath, I. Regupathi and M. B. Saidutta, Fuel Process. Technol., 2011, 92, 100–105 CrossRef PubMed.
- H. P. Li and H. Zhao, Energy Sources, 2015, 37, 334–340 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Calibration, OFAT and optimisation data. See DOI: 10.1039/c5ra15834f |
| This journal is © The Royal Society of Chemistry 2015 |