Spin-canting magnetization in 3D metal organic frameworks based on strip-shaped Δ-chains†
Xiu-Mei Zhang*,
Peng Li,
Wei Gao and
Jie-Ping Liu
College of Chemistry and Materials Science, Huaibei Normal University, Anhui 235000, China. E-mail: zhangxiumeilb@126.com
First published on 3rd September 2015
Abstract
Three isostructural coordination polymers, M2(TZI)(OH)(H2O)2·xH2O (x = 3, M = Cu(II) 1, x = 4, M = Co(II) 2 and Ni(II) 3) (H3TZI = 5-(1H-tetrazol-5-yl)isophthalic acid), have been synthesized under hydrothermal conditions. The compounds consist of 3D frameworks, in which the magnetic Δ-chain motifs based on corner-sharing M3(μ3-OH) isosceles triangle are linked by the TZI ligands, and they represent the rare (3,4,5,6)-connected 4-nodal net topology with the point symbol (43)(4462)(4664)(4768). Magnetic analyses indicate that compound 1 shows the coexistence of spin canting, metamagnetism and antiferromagnetic ordering, whereas compounds 2 and 3 exhibit canted antiferromagnetic coupling without magnetic ordering down to 2 K. Such magnetic behaviors above 2 K are still rare in Cu(II), Co(II) and Ni(II) compounds with similar chains.
Introduction
Molecular magnetism has attracted much attention in recent years due to its great value in understanding fundamental magnetic phenomena, revealing magnetostructural relationships, and constructing new molecular magnetic materials with potential technological applications.1–3 In particular, studies have been much promoted by the discoveries of magnetic materials with spontaneous magnetization, such as spin canting, metamagnetism and long-range magnetic ordering. It is well-known that a spin canting system can arise from two mechanisms: single-ion anisotropy and the antisymmetric Dzyaloshinskii–Moriya (DM) interaction.4 They both require that there is no inversion center between the interacting spins. Therefore, the antiferromagnetic (AFM) and ferromagnetic (FM) coupling between the noncollinear alignment of the spins usually result in spin-canting magnetic behaviors.5 Metamagnetism requires that the chain/layer must have net moments and may be FM or ferrimagnetic (FIM) or AFM with spin canting, and the interchain/interlayer interactions can induce AF ordering and are to be overcome by a critical field.6 Magnetic materials, especially, those displaying canted metamagnetic ordering, are viewed as one of the good candidates of molecular magnets. However, the design and synthesis of such magnetic materials are still a challenging task. The selection of the short bridging ligands between paramagnetic centers is very important; because they may transmit magnetic coupling and build the secondary building units (SBUs) to construct novel structural topology. The tetrazole and carboxylate as short bridging ligands have attracted much attention due to their coordinative and magnetic versatility. They can bind metal ions in various bridging modes and efficiently induce either FM or AFM coupling.7,8 To date, several Co(II) molecular magnets with tetrazole and μ3-OH bridged magnetic Δ-chains have been synthesized, including metamagnetism, spin-canting and long-range ordered systems.9 But the only one known Cu(II) species10 with the similar chains has been reported, which displays spin-frustrated antiferromagnetic ordering. We are interested in 5-(1H-tetrazol-5-yl)isophthalic acid (H3TZI), which has several remarkable features as follows: (i) two carboxylate groups may bind to metal centers with various coordination modes, allowing for varied magnetic interactions; (ii) the tetrazolate groups are expected to construct frustrated triangular motifs; (iii) both the carboxylate and tetrazolate groups of H3TZI have the ability to connect metal ions into high dimensional networks. Despite a few compounds with this ligand have been reported,11 it remains largely unexplored. Here we report three isostructural coordination polymers with this ligand, M2(TZI)(OH)(H2O)2·xH2O (x = 3, M = Cu(II) 1, x = 4, M = Co(II) 2 and Ni(II) 3) (H3TZI = 5-(1H-tetrazol-5-yl)isophthalic acid), in which the strip-shaped Δ-chains motifs built from corner-sharing M3(μ3-OH) triangle units with mixed double (μ4-tetrazolate)(μ3-OH) bridges. Compounds represent the rare (3,4,5,6)-connected 4-nodal net topology with the point symbol (43)(4462)(4664)(4768). Magnetic investigations indicated that they all display intrachain spin canting AFM interactions through the mixed double bridges but the bulk behaviors are different. Compound 1 exhibit spin-canted ordering and metamagnetism, whereas compounds 2 and 3 exhibit canted antiferromagnetic coupling without magnetic ordering down to 2 K, respectively. Noticeably, the complex magnetic phenomena of 1, 2 and 3 are different from previous compounds with similar chains.Experimental
Materials and physical measurements
All the solvents and reagents including 5-(1H-tetrazol-5-yl)isophthalic acid (H3TZI) were purchased commercially and were used as received. Infrared spectra were recorded on a NEXUS 670 FT-IR spectrometer using the KBr pellets. Elemental analysis was carried out in the range 500–4000 cm−1 on an Elementar Vario El III elemental analyzer. The phase purity of the samples was confirmed by powder X-ray diffraction collected on a Bruker D8-ADVANCE diffractometer equipped with Cu Kα at a scan speed of 1° min−1. Temperature-dependent and field-dependent magnetic measurements were carried out on a Quantum Design SQUID MPMS-5 magnetometer. Diamagnetic corrections were made with Pascal's constants.Crystal structure analysis
Diffraction data for 1 and 2 were collected at 293 K on a Bruker Apex II CCD area detector equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Empirical absorption corrections were applied using the SADABS program.12 The structures were solved by the direct method and refined by the full-matrix least-squares method on F2, with all non-hydrogen atoms refined with anisotropic thermal parameters.13 All the hydrogen atoms attached to carbon atoms were placed in calculated positions and refined using the riding model. The coordinated water hydrogen atoms were located from the difference maps. The uncoordinated water hydrogen atoms could not be modeled owing to the disorder and the limited quality of dataset. A summary of the crystallographic data, data collection, and refinement parameters are provided in Table 1.| Compounds | 1 | 2 |
|---|---|---|
| Empirical formula | Cu2C9H14N4O10 | Co2C9H16N4O11 |
| Formula weight | 465.32 | 474.12 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/m | P21/m |
| a/Å | 10.0150(9) | 10.1792(15) |
| b/Å | 6.6124(5) | 6.7279(9) |
| c/Å | 12.4721(11) | 12.8158(18) |
| α/° | 90 | 90 |
| β/° | 109.412(2) | 111.698(4) |
| γ/° | 90 | 90 |
| V/Å3 | 778.99(11) | 815.5(2) |
| Z | 2 | 2 |
| Dc (g m−3) | 1.984 | 1.931 |
| μ (mm−1) | 3.977 | 2.102 |
| F(000) | 468 | 480 |
| Reflections collected | 2553 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
| Unique reflections | 1449 | 2013 |
| GOF on F2 | 1.002 | 1.025 |
| Rint | 0.0877 | 0.0236 |
| R1 [I > 2σ(I)] | 0.1050 | 0.0345 |
| wR2 (all data) | 0.2989 | 0.0983 |
Results and discussion
Synthesis
Compounds 1–3 were synthesized by the reactions of metal(II) nitrate and H3TZI ligand in mixed aqueous acetonitrile at 150 °C. However, a Cu(II) MOF with TZI (ref. 11a) was obtained by the reactions of copper(II) nitrate and H3TZI ligand in an N,N-dimethylformamide/ethanol solution at 80 °C. The compositions and structures in 1–3 are quite different from those for a Cu(II) compound with ref. 11a. Notably, we report the first examples of novel coordination modes of this ligand. The coordination modes of the TZI ligand for the previous compounds have been given elsewhere.11The IR spectra of 1–3 show a broad absorption band at 3415–3457 cm−1, which should be ascribed to the ν(O–H) vibration of free and coordinated water molecules and the hydroxy groups. All compounds exhibit characteristic asymmetric (νas) and symmetric (νs) absorptions of the carboxylate groups14 and the tetrazole groups absorption peaks at 1400–1500 cm−1.15
Description of the structures
The central hydroxyl group uses its oxygen atom binding three M ions, generating an isosceles triangle [M3(μ3-OH)] with the M1⋯M2 distances of 3.562(2) and 3.5869(6) Å, and M2⋯M2 of 3.3062(3) and 3.3640(4) Å for 1 and 2, respectively, and M–O–M angle of about 117.0–117.4° for 1 for and 108.9–119.4° for 2. The μ3-OH oxygen atom is out of the mean basal plane by 0.3472(3) Å for 1 and 0.4223(3) Å for 2, which results in a non-coplanar [M3(μ3-OH)] triangle. The [M3(μ3-OH)] triangles are linked by sharing the M2 ions and adjacent M1 ions are bridged by μ-N1,N1F-tetrazolate groups to form an infinite strip-shaped Δ-chain along the b direction (Fig. 1b). It should be noted that a few examples of such Δ-chain topology have been reported so far.9,10 The Δ-chains in 1 and 2 were interlinked by TZI ligands to generate the 3D network framework with the shortest interchain M⋯M separations spanned by the TZI ligands being 7.907(4) Å and 8.141(1) Å, respectively (Fig. 2a). Each TZI ligand serves as a μ6-bridging mode, with the chelated carboxylate group binding one M1 ion and the other carboxylate group binding one symmetry related M1 ion in a monocarboxylate mode, meanwhile, the tetrazolate group bridging four metal ions (one pair of M1 and one pair of M2). The uncoordinated oxygen atom (O3A) of the monodentate carboxylate group hydrogen-bonded to a μ3-OH group oxygen atom (O5) with O5-H5B⋯O3A = 148.6(8)°, H5B⋯O3A = 1.974(2) Å and O5⋯O3A = 2.736(2) Å for 1; O5-H5B⋯O3A = 167.9(2)°, H5B⋯O3A = 2.136(6) Å and O5⋯O3A = 2.792(6) Å for 2.
 | ||
| Fig. 2 (a) A view of the 3D structure of 1. (b) View of 3D (3,4,5,6)-connected net with (43)(4462)(4664)(4768) topology for 1 (Cu1 turquiose, Cu2 green, O red, TZI blue). | ||
From the view of topology, the μ3-OH group and TZI ligand serve as 3- and 6-connected nodes, respectively, to join three M(II) ions (one M1 and two M2) and six M(II) ions (four M1 and two M2). The M1 and M2 play the 5- and 4-connected role, respectively, to link the TZI nodes and μ3-OH nodes (2 tetrazolate + 2 carboxylate + 1 μ3-OH nodes for M1 and 2 tetrazolate + 2 μ3-OH nodes for M2). Thus, the overall 3D network could be described as a 4-nodal (3,4,5,6)-connected net with the point symbol of (43)(4462)(4768) (Fig. 2b).
Magnetic properties
In order to confirm the actual coupling nature for compound 1, the zero-field-cooled (ZFC) and field-cooled (FC) magnetization were measured under 20 Oe in the range of 2–20 K (Fig. 3b). The FC and ZFC are identical and show a peak at 2.5 K, implying the short-range order of spins in antiferromagnetic coupling. Furthermore, the ac susceptibility shows a frequency-independent maximum at 6 K in the real (χ′) component (Fig. 3c), and no signal was observed in imaginary component (χ′′), supporting the onset of antiferromagnetic ordering below Tc = 2.6 K.
The isothermal magnetization curve at 2 K first increases rapidly and then increases slowly to 0.61 Nβ at 50 kOe with increased field, which is much lower than the saturation value of two Cu(II) ions (2.00 Nβ) (Fig. 4a), confirming a canted antiferromagnet. The linear region in high field was extrapolated to zero field, which gives a magnetization value of 0.415 Nβ. That value could be taken as the weak magnetization (Mr) contribution arising from spin canting. Thus, the canting angle (γ) can be roughly estimated by sin(γ) = Mr/MS to be 11.98°(MS = 2.0 Nβ for two Cu(II) ions).1a In the low-field region, the magnetization curve presents a sigmoid shape, which indicates a metamagnetic behavior. The critical field is estimated to be about 1.7 kOe according to the dM/dH derivative plot (Fig. 4a). Metamagnetism is also confirmed by the field-cooled (FC) magnetizations under different fields (Fig. 4b). The FC plot displays a maximum under 1.5 kOe at about 2.3 K, supporting the occurrence of antiferromagnetic ordering. As the field is lifted, the maximum shifts toward lower temperatures and becomes less prominent. When the external field is increased to 2.5 kOe, the maximum disappears, indicating that the antiferromagnetic interactions were overcome by a high field.16 The magnetization loop was measured by cycling of the field between −50 and 50 kOe at 2 K. No hysteresis was observed upon cycling of the field between −50 and 50 kOe at 2 K (Fig. S3†).
 | ||
| Fig. 4 (a) Isothermal magnetization curves at 2 K for 1; (b) FC magnetization curves for 1 at different fields. | ||
According to the structural data, Cu1 assumes an axially compressed octahedron, while Cu2 is an axially elongated octahedron. So, the unpaired electron is in dz2 orbital of Cu1 but in dx2 − y2 of Cu2. So that it is ferromagnetic coupling between Cu1 and Cu2 ions. It is well known that the interaction in the Cu(II) systems is sensitive to the Cu–O–Cu angle, with large bridging angles (>97.5°) transmitting antiferromagnetic coupling.17 Thus, O5 and tetrazole ligand mediate the antiferromagnetic coupling between Cu2 ions, because 1 has a larger Cu2–O–Cu2 angle [117.37°] in equatorial plane and tetrazole provides a two – atoms bridge mediating antiferromagnetic interaction in spin polarization mechanism. The interaction intrachain between Cu1 ions is very weak by tetrazole groups, which can be neglected. The antiferromagnetic coupling is much stronger than the ferromagnetic coupling, so the spin at Cu1 is frustrated. Just for this reason, this system shows the spin-canting phenomenon. The canting source is from Cu1 and the canting angle should be larger than the usual antiferromagnetic system.
The magnetic susceptibility (χ) of 2 is shown in Fig. 5a. The χT value per Co2 at room temperature is about 6.42 emu K mol−1, which is much larger than the spin-only value of 3.75 emu K mol−1 for three non-interacting octahedral Co(II) ions (S = 3/2, g = 2.00), owing to the strong orbital contribution. As the temperature is lowered, the χT value decreases continuously, while the χ value increases slowly to an approximate plateau at about 9 K and then increases rapidly upon further cooling to 2 K. This shape of the plots may be attributed to spin canting, due to the alignment of these antiferromagnetic interactions in an isosceles triangle. The data above 200 K follow the Curie–Weiss law with C = 6.72 emu K mol−1 and θ = −14.3 K. The decrease of χT with decreased temperature indicates antiferromagnetic coupling between Co(II) ions. But the negative θ value is not necessary for the antiferromagnetic interactions because a single octahedral Co(II) ion has the effects of the spin–orbital coupling. The isothermal magnetization curve measured at 2 K (Fig. 5b) also supports the spin-canting of 2. The magnetization first increases rapidly and then increases slowly with the 0.75 Nβ at 50 kOe with increased field, which is much lower than the saturation value of two Co(II) ions. These features are consistent with the antiferromagnetic interactions in the intrachain. The nonlinearity of the low-field range can be due to spin canting. Thermal ac susceptibilities were measured on 2 under a zero dc field at different frequencies (Fig. S4†). The real (χ′) component exhibits a frequency-independent without the maximum and no signal was observed the imaginary component (χ′′), suggesting magnetic ordering not occurring above 2 K. The intrachain magnetic behaviors are similar to those of previous compounds with similar Δ-chains based on mixed double bridges (μ3-OH and μ4-tetrazolate),9 which also are ascribed to the spin canting antiferromagnetic coupling.
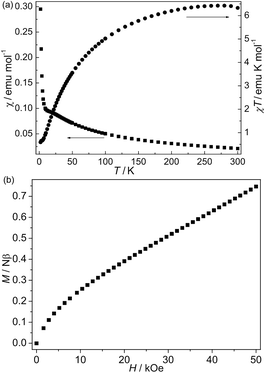 | ||
| Fig. 5 (a) The χT and χ vs. T plot of 2 at 1 kOe; (b) field-dependent isothermal magnetization curve of 2 at 2 K. | ||
The overall magnetic behavior of 3 is similar to that of 2. The χT value per Ni2 at 300 K is 2.54 emu K mol−1, corresponding to a S = 1 spin with g > 2 (Fig. 6a). Upon cooling, the χT value decreases continuously, while the χ value increases slowly to an approximate plateau and then increases rapidly upon further cooling to 2 K. This behavior is characteristic of a canted spin system. The data above 120 K follow the Curie–Weiss law with C = 2.92 emu K mol−1 and θ = −44.3 K. The negative value of θ indicates antiferromagnetic coupling in the high temperature range. The magnetization curve increases nonlinearly with the 0.32 Nβ at 50 kOe with increased field (Fig. 6b), which is far from the saturation value, in agreement with that expected for a spin-canted antiferromagnet. Frequency independent behavior was observed in thermal ac susceptibility curves (Fig. S5†). No maximum in the real component (χ′) and no imaginary signal (χ′′) were observed, indicating the antiferromagnetic interaction between Ni(II) ions and the absence of long-range ordering above 2 K.
 | ||
| Fig. 6 (a) The χT and χ vs. T plot of 3 at 1 kOe; (b) field-dependent isothermal magnetization curve of 3 at 2 K. | ||
Spin canting can be constructed by using Δ-chain containing triangle motifs with antisymmetric magnetic exchange and/or single-ion magnetic anisotropy. The isostructural compounds 1–3 with magnetic Δ-chain all exhibit intrachain spin canted antiferromagnetic interactions but show distinct bulk properties: compound 1 shows spin canting, antiferromagnetic ordering and field-induced metamagnetism, whereas compounds 2 and 3 exhibit canted antiferromagnetic coupling without magnetic ordering above 2 K. The canted antiferromagnetic coupling in 1 arised from the competition of antiferromagnetic and ferromagnetic interactions between spins, which results in uncompensated residual spins. The spin-competing antiferromagnetic interaction is presented in this system due to the magnetic Δ-chains. As for 2 and 3, the Co(II) and Ni(II) ions have spin–orbital coupling and single-ion anisotropy. The spin–orbital coupling destroys the competing interaction in the system, and results in spin canting, and single-ion anisotropy (one of the origins of spin-canting) of Co(II) ion favours spin canting. The occurrence of 3D magnetic ordering may be related to the intrachain and/or interchain interactions. Generally, the intrachain factors can determine the occurrence of ordering and the ordering temperature.18 The ordering temperature can also be evoked by interchain exchange and the degree of spin canting. Two interchain interactions are essential for magnetic ordering: one is the superexchange interactions interchains through weak bonds, which disappears very rapidly as the distance increases, and the other is dipolar interactions interchains through space, which has long-range effects. The magnetic difference among compounds 1–3 is related to interchain and/or intrachain interaction. It is difficult to make detailed comparisons between the two series owing to the wide different nature of bridges and spin centers.
Conclusions
Three new spin canting magnets with corner-sharing M3(μ3-OH) isosceles triangle Δ-chain were synthesized and structurally and magnetically characterized. The isostructural compounds 1–3 show 3D structures assembled by the TZI (H3TZI = 5-(1H-tetrazol-5-yl)isophthalic acid) ligands linking Δ-chains, which represent the rare (3,4,5,6)-connected 4-nodal net topology with the point symbol (43)(4462)(4664)(4768). Magnetic investigations on three compounds reveal that all exhibit intrachain canted antiferromagnetic interactions. 1 shows the coexistence of spin canting, metamagnetism and antiferromagnetic ordering, whereas 2 and 3 exhibit canted antiferromagnetic coupling without magnetic ordering above 2 K. More interesting, the coexistence of several magnetic behaviors was observed in 1, and the origin of spin canting was discussed. Especially, compounds 1 and 3 are the first Cu(II) and Ni(II) compounds with such magnetic behaviors above 2 K in the series. This work provide a good example for magnetic studies with similar structures and different spin carriers to research magneto-structural correlations.Acknowledgements
We are thankful for the financial support from NSFC (21201069) and the Natural Science Foundation of Anhui Province (1308085QB23).Notes and references
- (a) O. Kahn, Molecular Magnetism, VCH, Weinheim, Germany, 1993 Search PubMed; (b) J. S. Miller, Adv. Mater., 2002, 14, 1105 CrossRef CAS; (c) J. S. Miller and M. Drillon, Magnetism: Molecules to Materials, Wiley-VCH, Weinheim, Germany, 2002−2005, vol. I−V Search PubMed; (d) R. L. Carlin. Magnetochemistry, Springer, Berlin-Heidelberg, 1986 Search PubMed; (e) C. Coulon, H. Miyasaka and R. Clérac, Struct. Bonding, 2006, 122, 163 CrossRef CAS.
- (a) D. F. Weng, Z. M. Wang and S. Gao, Chem. Soc. Rev., 2011, 40, 3157 RSC; (b) J. R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477 RSC; (c) P. Zhang, Y. N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef CAS PubMed; (d) X. Y. Wang, C. Avendano and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213 RSC.
- (a) Y. Hu, M. Zeng, K. Zhang, S. Hu, F. Zhou and M. Kurmoo, J. Am. Chem. Soc., 2013, 135, 7901 CrossRef CAS PubMed; (b) S. Xiang, X. Wu, J. Zhang, R. Fu, S. Hu and X. Zhang, J. Am. Chem. Soc., 2005, 127, 16352 CrossRef CAS PubMed; (c) P. L. Arnold, Nat. Chem., 2012, 4, 967 CrossRef CAS PubMed; (d) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed.
- (a) T. Moriya, Phys. Rev., 1960, 117, 635 CrossRef CAS; (b) I. Dzyaloshinsky, J. Phys. Chem. Solids, 1958, 4, 241 CrossRef CAS; (c) T. Moriya, Phys. Rev., 1960, 120, 91 CrossRef CAS.
- (a) M. B. Hastings, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 014413/1 CAS; (b) D. Shao, S.-L. Zhang, X.-H. Zhao and X.-Y. Wang, Chem. Commun., 2015, 51, 4360 RSC; (c) B. Li, Z. Li, R.-J. Wei, F. Yu, X. Chen, Y.-P. Xie, T.-L. Zhang and J. Tao, Inorg. Chem., 2015, 54, 3331 CrossRef CAS PubMed; (d) B. M. Bartlett and D. G. Nocera, J. Am. Chem. Soc., 2005, 127, 8985 CrossRef CAS PubMed; (e) D. Papoutsakis, D. Grohol and D. G. Nocera, J. Am. Chem. Soc., 2002, 124, 2647 CrossRef CAS PubMed.
- (a) B. Deviren and M. Keskin, Phys. Lett. A, 2010, 374, 3119 CrossRef CAS PubMed; (b) N. Motokawa, S. Matsunaga, S. Takaishi, H. Miyasaka, M. Yamashita and K. R. Dunbar, J. Am. Chem. Soc., 2010, 132, 11943 CrossRef CAS; (c) W.-W. Sun, C.-Y. Tian, X.-H. Jing, Y.-Q. Wang and E.-Q. Gao, Chem. Commun., 2009, 4741 RSC; (d) X.-M. Zhang, Y.-Q. Wang, Y. Song and E.-Q. Gao, Inorg. Chem., 2011, 50, 7284 CrossRef CAS PubMed.
- (a) W. Ouellette, A. V. Prosvirin, K. Whitenack, K. R. Dunbar and J. Zubieta, Angew. Chem., 2009, 121, 2174 (Angew. Chem., Int. Ed., 2009, 48, 2140) CrossRef PubMed; (b) M. Dinca, A. F. Yu and J. R. Long, J. Am. Chem. Soc., 2006, 128, 8904 CrossRef CAS PubMed; (c) G. Aromí, L. A. Barrios, O. Roubeau and P. Gamez, Coord. Chem. Rev., 2011, 255, 485 CrossRef PubMed; (d) X.-B. Li, J.-Y. Zhang, Y.-Q. Wang, Y. Song and E.-Q. Gao, Chem.–Eur. J., 2011, 17, 13883 CrossRef CAS PubMed; (e) X.-B. Li, G.-M. Zhuang, X. Wang, K. Wang and E.-Q. Gao, Chem. Commun., 2013, 49, 1814 RSC; (f) Q.-X. Jia, Y.-Q. Wang, Q. Yue, Q.-L. Wang and E.-Q. Gao, Chem. Commun., 2008, 4894 RSC; (g) X.-M. Zhang, P. Li, W. Gao, J.-P. Liu and E.-Q. Gao, Dalton Trans., 2015, 511 RSC.
- (a) H. Tian, Q.-X. Jia, E.-Q. Gao and Q.-L. Wang, Chem. Commun., 2010, 46, 5349 RSC; (b) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC; (c) Q. Sun, A.-L. Cheng, Y.-Q. Wang, Y. Ma and E.-Q. Gao, Inorg. Chem., 2011, 50, 8144 CrossRef CAS PubMed; (d) Y.-Z. Zheng, M.-L. Tong, W. X. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2006, 45, 6310 CrossRef CAS PubMed; (e) S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870 CrossRef CAS PubMed; (f) E. A. Nytko, J. S. Helton, P. Müller and D. G. Nocera, J. Am. Chem. Soc., 2008, 130, 2922 CrossRef CAS PubMed.
- (a) R.-X. Yao, Y.-L. Qin, F. Ji, Y.-F. Zhao and X.-M. Zhang, Dalton Trans., 2013, 6611 RSC; (b) W.-C. Song, J. Tao, T.-L. Hu, Y.-F. Zeng and X.-H. Bu, Dalton Trans., 2011, 11955 RSC; (c) E.-C. Yang, Z.-Y. Liu, X.-Y. Wu and X.-J. Zhao, Chem. Commun., 2011, 47, 8629 RSC; (d) D.-S. Liu, Y. Sui, T.-W. Wang, C.-C. Huang, J.-Z. Chen and X.-Z. You, Dalton Trans., 2012, 5301 RSC.
- E.-C. Yang, Z.-Y. Liu, X.-Y. Wu, H. Chang, E.-C. Wang and X.-J. Zhao, Dalton Trans., 2011, 10082 RSC.
- (a) F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833 CrossRef CAS PubMed; (b) A. J. Calahorro, A. Salinas-Castillo, J. M. Seco, J. Zuñiga, E. Colacioa and A. Rodríguez-Diéguez, CrystEngComm, 2013, 15, 7636 RSC; (c) Z.-R. Qu, Z. Xing, B.-Z. Wu, X.-Z. Li and G.-F. Han, Z. Anorg. Allg. Chem., 2009, 635, 39 CrossRef CAS PubMed; (d) J. Jia, J.-N. Xu, S.-Y. Wang, P.-C. Wang, L.-J. Gao, M. Yu, Y. Fan and L. Wang, CrystEngComm, 2015, 17, 6030 RSC.
- G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, SHELXTL Version 5.1, Bruker Analytical X-ray Instruments Inc., Madison, Wisconsin, USA, 1998 Search PubMed.
- (a) K. Nakamoto, Infrared and Raman Spectra of Inorganic and coordination Compounds Part B, John Wiley, New York, 5th edn, 1997 Search PubMed; (b) G. B. Deacon and R. J. Phillips, Coord. Chem. Rev., 1980, 33, 227 CrossRef CAS.
- (a) Y. C. Qiu, H. Deng, J. X. Mou, S. H. Yang, M. Zeller, S. R. Batten, H. H. Wu and J. Li, Chem. Commun., 2009, 5415 RSC; (b) Y. C. Qiu, Y. H. Li, G. Peng, J. B. Cai, L. M. Jin, L. Ma, H. Deng, M. Zeller and S. R. Batten, Cryst. Growth Des., 2010, 10, 1332 CrossRef CAS; (c) B. Liu, Y. C. Qiu, G. Peng and H. Deng, CrystEngComm, 2010, 12, 270 RSC.
- R. L. Carlin. Magnetochemistry, Springer, Berlin, 1986 Search PubMed.
- (a) R. W. Jotham and S. F. A. Kettle, Inorg. Chim. Acta, 1970, 4, 145 CrossRef CAS; (b) Y.-f. Song, C. Massera, O. Roubeau, P. Gamez, A. M. M. Lanfredi and J. Reedijk, Inorg. Chem., 2004, 43, 6842 CrossRef CAS PubMed; (c) V. H. Crawford, H. W. Richardson, J. R. Wasson, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 1976, 15, 2107 CrossRef CAS.
- (a) J. S. Miller, Chem. Soc. Rev., 2011, 40, 3266 RSC; (b) M. Drillon and P. Panissod, J. Magn. Magn. Mater., 1998, 188, 93 CrossRef CAS; (c) P. Panissod and M. Drillon, in Magnetism-From Molecules to Materials, ed. J. S. Miller and M. Drillon, Wiley-VCH, Weinheim, 2002, vol. IV, p. 233269 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S5. CCDC 1409334 and 1409333. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra15937g |
| This journal is © The Royal Society of Chemistry 2015 |


