Thermal curing of novel carborane-containing phenylethynyl terminated imide oligomers†
Abstract
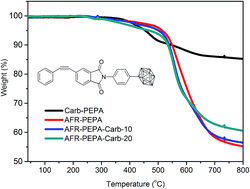
A novel carborane-containing phenylethynyl terminated imide compound (Carb-PEPA) as a modifier for fluorinated phenylethynyl terminated imide oligomer (AFR-PEPA) was synthesized and characterized by FT-IR and 1H-NMR. The resin systems consisting of Carb-PEPA and AFR-PEPA (AFR-PEPA-Carb) were prepared with different weight fractions of Carb-PEPA. The thermal curing kinetics and thermal stability of the imide compound and resultant resin systems were analyzed by using DSC and TGA respectively. The relevant kinetic data were determined by a Kissinger method. The results show that the glass transition temperatures (Tg) of the cured resin systems increase with the addition of Carb-PEPA. The imide system containing 20 wt% Carb-PEPA (AFR-PEPA-Carb-20) exhibits the highest Tg of 389.5 °C due to the high steric hindrance of carborane. The char yield of the resin was also increased with the introduction of the carborane structure in the system. The kinetic data indicate that AFR-PEPA/Carb-PEPA imide blends have higher activation energy E and frequency factor A than AFR-PEPA imide oligomers.

 Please wait while we load your content...
Please wait while we load your content...