Swelling technique inspired synthesis of a fluorescent composite sensor for highly selective detection of bifenthrin†
Abstract
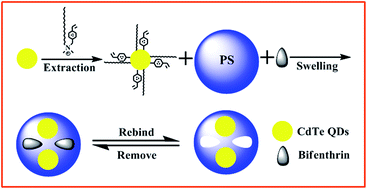
Pesticide pollution has become a serious problem that threatens public health, so it is necessary to develop a method that can detect pesticides rapidly and sensitively. In this study, we report a novel fluorescent imprinted sensor based on quantum dots (QDs) synthesized via a facile and versatile swelling technique for highly selective detection of bifenthrin (BI). Compared with other fluorescent molecularly imprinted polymers (MIPs), it has three significant differences: firstly, polystyrene (PS) microspheres make up the polymer matrix and were prepared in advance; secondly, the interactions are not hydrogen bonding and covalent interactions, but van der Waals and hydrophobic forces; thirdly, aqueous QDs were successful applied to the swelling process using a polymerizable surfactant. The unique fluorescent sensor (MIPs (PS)-OVDAC/CdTe QDs) possesses the strong fluorescence and sensitivity of QDs and the high selectivity of molecularly imprinted polymers as well as a uniform morphology via this novel swelling strategy. As a result, the fluorescence intensity of the MIPs (PS)-OVDAC/CdTe QDs was strongly decreased within less than 25 min upon binding BI, and the quenching fractions of the MIPs (PS)-OVDAC/CdTe QDs presented a good linearity with BI concentrations in the range of 0.5–40 μM with a correlation coefficient of 0.9918. In addition, the limit of detection (LOD) was as low as 0.08 μmol L−1 and a high imprinting factor of 4.11 was obtained. The developed method was successfully applied to the determination of BI in honey samples. The present study provides a facile and efficient strategy to develop fluorescent sensors for rapid recognition and selective detection of organic pollutants from complex matrices.

 Please wait while we load your content...
Please wait while we load your content...