A simple and reversible fluorescent probe based on NBD for rapid detection of hypochlorite and its application for bioimaging†
Abstract
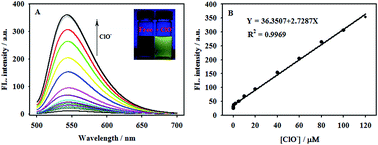
A simple and reversible fluorescent probe bearing 7-nitrobenz-2-oxa-1,3-diazole and a selenomorpholine fragment was designed and synthesized. The probe showed highly selective, sensitive and fast (<10 s) recognition to hypochlorite in aqueous solutions. The relative results demonstrated that the linear response range of the probe was between 5.0 × 10−8 M and 1.2 × 10−4 M, with a low detection limit of 3.3 nM (S/N = 3). The probe was capable of monitoring hypochlorite reversibly in the presence of glutathione. In addition, the biological applications in living cells have been described.

 Please wait while we load your content...
Please wait while we load your content...