Structural and physico-chemical properties of insoluble rice bran fiber: effect of acid–base induced modifications
Jing Qia,
Wallace Yokoyamab,
Kingsley George Masambaa,
Hamid Majeeda,
Fang Zhong*a and
Yue Li*a
aKey Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Food Science and Technology, Jiangnan University, Wuxi 214122, P. R. China. E-mail: fzhong@jiangnan.edu.cn; liyue@jiangnan.edu.cn; Fax: +86-510-85197876; Tel: +86-510-85197876
bWestern Regional Research Center, ARS, USDA, Albany, CA 94710, USA
First published on 10th September 2015
Abstract
The structural modifications of insoluble rice bran fiber (IRBF) by sequential regimes of sulphuric acid (H2SO4) and their effects on the physicochemical attributes were studied. The increment of H2SO4 concentration resulted in decreased water holding capacity that ultimately enhanced the oil binding capacity due to the partial removal of starch, protein and hemicelluloses. The starch and hemicelluloses were hydrolyzed exponentially by sequential increments of H2SO4 while protein was mainly dissolved by KOH for all samples. Moreover, higher H2SO4 concentration improved the porosity and crystallinity that led to higher thermal stability of the fiber as evident from XRD and TGA analysis. Furthermore, decreased monosaccharide linkages and increases of porosity with H2SO4 regimes were confirmed by FT-IR and SEM. The change in composition and microstructure of insoluble rice bran fiber (IRBF) induced significant physicochemical changes that might be suitable for their application in the food industry as an anti-diabetic and cholesterol lowering functional ingredient.
Introduction
The intake of whole grains has been shown to reduce the risk of diabetes and cardiovascular disease in population studies due to protective factors including dietary fiber, vitamin E and other nutrients.1,2 Most of the dietary fiber from plant sources, including cereal brans, is classified as insoluble dietary fiber (IDF). The IDF of cereal bran is mainly composed of cellulose, hemicelluloses and lignin that contain several functional groups such as alcohols, aldehydes, ketones, carboxylic acid, phenolic and ether linkages.3 These groups have a strong affinity to bind water, oil or toxic metal ions. However, in order to use these cereal brans,4,5 they require some level of pre-treatment that helps to expose the binding sites or increase the porosity. Therefore, several common physical and chemical pretreatments such as micronization,6 enzymatic treatment7 as well as some inorganic and organic bases, acids and salt solutions3 treatments have been reported. The extent of physical pretreatment strongly depends on particle size while enzyme processes usually require complex steps and high dosage of costly enzymes as well as precise regulation of reaction temperature. In many instances, acidic pretreatment is found more successful, primarily due to easy removal of impurities and ions that might block the functional groups or porous structures.3,8,9Rice bran (RB) is a byproduct of rice milling, and is left over in large quantity by the rice industry every year. In recent years, most of rice bran is used as animal feed ingredient, fertilizer and fuel.10 But there is an underestimated potential for high-value rice bran production because of its high content of IDF. Rice bran (RB) is composed of about 27% DF7 and almost 90% of the IDF accounts for rice bran DF as the main component.11 Though, a few studies for improving insoluble rice bran fiber (IRBF) functional properties such as fat binding and emulsifying capacity to stabilize emulsions of the food system7 or the adsorption capacity of fiber for the removal of Ni(II) from aqueous solution3 have been reported, in most of these studies, the use of rice bran was based on its granular structure, insolubility in water, chemical stability and local availability.
Since the chemical pre-treatments can potentially modify the cell surface either by removing or masking the groups or exposing more porous structure, therefore, we investigated the effect of inorganic acid concentration on the composition and microstructure that might expose various functional properties of IRBF. The present study aimed to simplify the extraction process, reduce the cost and improve the physicochemical properties of IRBF for food system enrichment, and to understand the relationship between microstructure and physicochemical properties of fiber. Various researchers reported acid induced modification of cereal fibers and further characterize their physicochemical attributes in terms of water holding capacity (WHC), oil binding capacity (OBC), swelling capacity (SWC) and cation-exchange capacity (CEC).12,13 In the present study we applied acid–base regimes on rice bran and evaluated their structural and physicochemical characteristics.
Experimental
Materials and reagents
Rice bran of paddy rice (non waxy rice) was collected from small rice processing mills situated in Wuxi city, Jiangsu province, China. The α-amylase (BAN 480 L) and protease (Neutrase 6.8 L) were supplied by Novozymes Biotechnology (Beijing, China) and were used as received. Other reagents used were of analytical grade.Pre-treatment of rice bran
The fresh rice bran was dried at 60 °C in a forced-air oven and screened through a 40-mesh sieve. Defatting was executed and conducted in duplicate by soaking the rice bran in n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, w/v) at room temperature for 12 h and then decanting the n-hexane. The defatted rice bran was first air dried in a fume hood to remove residual hexane and then dried at 60 °C in a forced-air oven and kept in sealed bags until used.
5, w/v) at room temperature for 12 h and then decanting the n-hexane. The defatted rice bran was first air dried in a fume hood to remove residual hexane and then dried at 60 °C in a forced-air oven and kept in sealed bags until used.
Chemical composition analysis of rice bran
Compositional properties of rice bran were determined using standard method of AOAC (2005).14 Moisture content (method 930.15) was determined by drying in an oven (DHG-9140A, YH Scientific instrument Co., LTD, Shanghai, China) at 105 °C until constant weight. Crude fat (method 920.39) was determined by the Soxhlet extraction method using petroleum ether as a solvent. Crude protein content (method 992.15) was determined by the Kjeldahl method with an automatic Kjeldahl nitrogen analyzer (KDN-103F, Xianjian instrument Co., LTD, Shanghai, China), using 6.25 as the conversion factor. Ash content (method 920.153) was determined using a Thermolyne Type 6000 muffle furnace (Thermo Scientific, Lawrence, KS) at 550 °C. Total carbohydrate content was calculated by difference. The determination of insoluble dietary fiber (IDF) was carried out according to the method of Yeh et al.15 and the soluble dietary fiber (SDF) content was calculated by subtracting the IDF from total carbohydrate content.Extraction of insoluble fiber from rice bran
The IRBF was isolated according to the method described by Unni et al.16 with minor modifications. The defatted rice bran was treated with boiling dilute H2SO4 solutions for 30 min with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) at different concentrations (0.2%, 0.4%, 0.6%, 0.8%, 1.0%, 1.25%, 1.5%, 2.0%, 3.0%, 4.0% and 5.0%, w/v) before treating with 1.25% KOH. The residue was filtered and washed with hot water until the pH of solution is neutral. The 11 residues were washed with ethanol and then petroleum ether followed by air-drying in a fume hood to remove organic solvents. Finally, the obtained samples were dried at 60 °C in a forced-air oven for 24 h and were ground with a high-speed universal grinder (DFY-200, Linda machinery Co., Zhejiang, China), screened through a 40 mesh sieve and kept in a desiccator for further analysis.
10 (w/v) at different concentrations (0.2%, 0.4%, 0.6%, 0.8%, 1.0%, 1.25%, 1.5%, 2.0%, 3.0%, 4.0% and 5.0%, w/v) before treating with 1.25% KOH. The residue was filtered and washed with hot water until the pH of solution is neutral. The 11 residues were washed with ethanol and then petroleum ether followed by air-drying in a fume hood to remove organic solvents. Finally, the obtained samples were dried at 60 °C in a forced-air oven for 24 h and were ground with a high-speed universal grinder (DFY-200, Linda machinery Co., Zhejiang, China), screened through a 40 mesh sieve and kept in a desiccator for further analysis.
Effect of various concentrations of acid treatment on composition of IRBF
Starch content was based on the monosaccharide method using 0.9 as the conversion factor.14 All weights and calculations were made on oven dried samples (60 °C, 24 h).Hemicellulose and cellulose contents were determined according to the procedure described by Sun et al.17 and Egüé et al.18 The fiber was dewaxed with methanol–chloroform (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) followed by air drying and hydrolysis (the ratio of solid to liquid was 1
2, v/v) followed by air drying and hydrolysis (the ratio of solid to liquid was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) with amylase and protease (pH = 6.5, 65 °C, 2 h) to remove starch and protein. The protein and starch free residue was delignified with sodium chlorite solution (pH = 4.0, 75 °C, 2 h) at a ratio of 1
40) with amylase and protease (pH = 6.5, 65 °C, 2 h) to remove starch and protein. The protein and starch free residue was delignified with sodium chlorite solution (pH = 4.0, 75 °C, 2 h) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 to obtain the holocellulose fraction. The hemicellulose was separated from the holocellulose by alkaline treatment (10% NaOH, 1
25 to obtain the holocellulose fraction. The hemicellulose was separated from the holocellulose by alkaline treatment (10% NaOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20; w/v, 10 h, 20 °C) to solubilize the hemicellulose, and the cellulose was filtered off. The hemicellulose was precipitated with three volumes of ethanol from the filtrate after acidification (pH = 5.5, adjusted by 6 M HCl). Both hemicellulose and cellulose were quantified after drying at 60 °C for 12 h.
20; w/v, 10 h, 20 °C) to solubilize the hemicellulose, and the cellulose was filtered off. The hemicellulose was precipitated with three volumes of ethanol from the filtrate after acidification (pH = 5.5, adjusted by 6 M HCl). Both hemicellulose and cellulose were quantified after drying at 60 °C for 12 h.
Measurement of water holding capacity (WHC) and oil binding capacity (OBC)
WHC and OBC of IRBF were determined according to the method of Sangnark and Noomhorm19 with some modifications. 1.0 g of dried samples was mixed with 70-fold (w/v) distilled water and allowed to equilibrate for 12 h. The excess water was removed by draining through a nylon mesh and the wet sample was collected, weighed (wet weight) and dried (105 °C) to constant weight (±0.05 mg, dry weight). WHC was defined as follows:
 | (1) |
For the OBC, 1 g of sample was combined with excess oil and equilibrated for 4 h. The excess oil was drawn off with a pipette and filter paper after centrifugation at 1500 × g for 20 min. The OBC was calculated as follows:
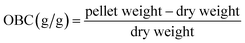 | (2) |
Measurement of bulk density and swelling capacity (SWC)
The bulk density was measured according to the method described by Chau et al.20 The sample was placed in a graduated cylinder without compaction. The bulk density was defined as follows:
 | (3) |
The determination of SWC was carried out according to the method reported by Navarro-González et al.21 with some modifications. The measurement was executed by transferring 0.5 g of samples into a calibrated cylinder and the bed volume was recorded. Then, 8 mL of distilled water was added. After hydrating for 5 h at room temperature, the swelling volume was recorded. The SWC was expressed as follows:
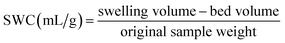 | (4) |
Measurement of cation-exchange capacity (CEC)
The CEC was measured according to the procedure described by Chau and Cheung22 with a slight modification. Briefly, 0.2 g of dried samples was converted into their acidic forms by stirring for 24 h at room temperature in 70-fold (w/v) 0.1 M hydrochloric acid followed by extensive washing until the filtrate was free from Cl− (verified against 10% AgNO3 solution). After vacuum drying (40 °C, 12 h), the activated powders were dispersed in 50 mL of 5% sodium chloride and were titrated with 0.02 M NaOH using phenolphthalein (2 g L−1) as an indicator. The CEC was expressed as the number of milliequivalents per gram of dry sample (meq g−1).Scanning electron microscopy (SEM)
The surface microstructure of raw material and three modified IRBFs with significant differences in crystallinity and thermal stability were selected for scanning electron microscope (Quanta-200, FEI Co., Netherland) observation with an accelerating potential of 5.0 kV and magnifications of 600 and 300. The treated rice bran powder was deposited on a metal stub, coated with a thin layer of gold (approximately 30 Å) in a vacuum for 30 s by an ion sputter. Then the surface and microstructure were observed by the SEM.X-ray diffraction (XRD) determination
The raw material and five representative samples treated with low, medium and high concentrations of H2SO4 (0.2, 0.8, 1.25, 2.0, 5.0%) followed by 1.25% KOH were selected for further study including XRD, thermogravimetric analysis (TGA) and Fourier transform infrared spectroscopy (FT-IR).The XRD determination was performed at room temperature with a voltage of 40 kV and a current of 40 mA using X-ray diffractometer (D8, Brucker AXS GMBH, Germany). The determination was executed using Cu-Kα radiation λ = 1.541 Å within the scanning range of 4–70° coupling with a scanning speed of 4° (2θ) min−1 and a scanning step of 0.02°.
The crystallinity indices (CrI) of the samples were calculated using the following equation:
 | (5) |
Thermogravimetric analysis (TGA)
The thermogravimetric (TG) and derivative thermogravimetric (DTG) analysis of the samples were evaluated using TGA/SDTA analyzer (TGA/SDTA851e, Mettler Toledo, Switzerland). Approximately 4 to 10 milligrams of the samples were weighed in an alumina crucible and heated at controlled temperatures from 25 °C up to 650 °C at a rate of 10 °C min−1. Nitrogen (99.9%) was employed as the carrier gas with a flow rate of 30 mL min−1.Fourier transform infrared spectroscopy (FT-IR)
FT-IR spectra were obtained at a resolution of 32 cm−1 in the range of 4000–400 cm−1 using a Nicolet iS10 FT-IR spectrophotometer (Thermo Fisher Scientific Inc., New York, USA). The ground samples were incorporated into spectroscopic grade KBr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, w/w) and pressed into a 1 mm pellet. The spectrum of pure KBr was used as background. Each spectrum was the average of 32 scans.
100, w/w) and pressed into a 1 mm pellet. The spectrum of pure KBr was used as background. Each spectrum was the average of 32 scans.
Statistical analysis
Each experiment for analyzing physicochemical properties of tested samples was conducted in triplicates and the data was analyzed by one-way analysis of variance (ANOVA) using the statistical package SPSS 19.0 (SPSS Inc., Chicago, IL.). The results were expressed as means ± standard deviations, and reported on a dry matter basis with a significance level of 95%.Results and discussion
Chemical composition of rice bran
The proximate composition of rice bran as dry weight is summarized in Table 1. The rice bran was found to be rich in total carbohydrate (46.4 g/100 g), and possessed low levels of moisture (7.4 g/100 g) and ash (4.9 g/100 g). The crude fat, protein and SDF contents of rice bran were around 20.1%, 21.2% and 18.9%, respectively. The IDF content of rice bran was in similar range as indicated by other researchers.7,23,24 However, its quantity was higher than that of other cereal and fruit byproducts such as oat bran, pear and orange (20.2–24.2 g/100 g).25 On behalf of compositional variations we can say that acid–base modified IRBF could be a promising source of dietary fiber with superior physicochemical attributes that might fulfill the demand of functional food.| Composition | g/100 g of pomace, dry weight |
|---|---|
| a The data is expressed as mean ± standard deviation. | |
| Moisture | 7.4 ± 0.02 |
| Crude fat | 20.1 ± 0.5 |
| Crude protein | 21.2 ± 1.6 |
| Ash | 4.9 ± 0.02 |
| Total carbohydrate | 46.4 ± 2.1 |
| Insoluble dietary fiber (IDF) | 27.5 ± 1.7 |
| Soluble dietary fiber (SDF) | 18.9 ± 0.4 |
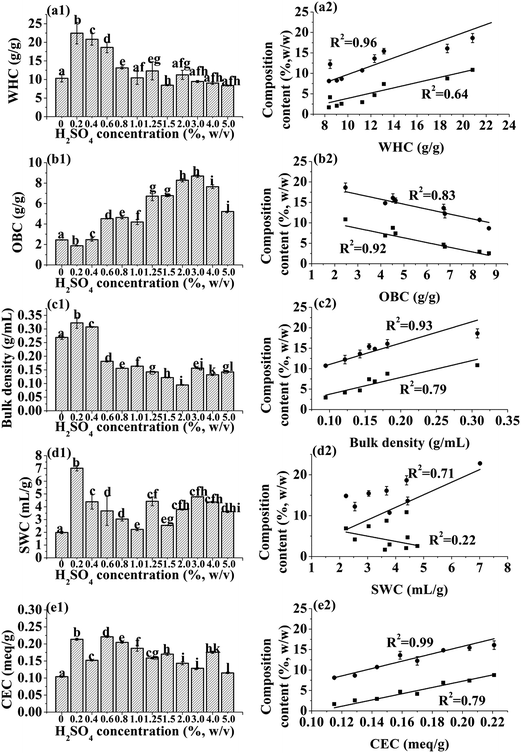
Effect of different acid concentrations on the compositions of IRBF
The compositional changes induced by successive acid hydrolysis of the defatted rice bran are shown in Fig. 1a. The contents of starch, hemicelluloses and protein decreased dramatically followed by a gradual decline with increasing H2SO4 concentration. The increase of acid concentration exponentially decreased the starch (R2 = 0.90) content by 47.1% and hemicelluloses content (R2 = 0.96) by 64.3%.The most diluted H2SO4 regime (0.2%) coupled with 1.25% KOH solubilized most of the protein to a constant value (less than 2% of the dry weight). The protein content showed negligible decrease even at concentrated H2SO4 regime (5%) and overall protein content remained 2.5% for all modified samples. It can be deduced that protein was mainly dissolved by base and the results obtained were comparable to enzymatic treatment as reported by Feng and Qiu.26
Fig. 1b showed exponential decrease in hemicellulose content with increasing acid concentration (R2 = 0.96). However, some hemicellulose remained at highest acid regime (5%) that might be due to covalent crosslinking with residual lignin and cellulose. The acid regimes sequentially removed starch, protein and hemicellulose that conversely increased the amount of cellulose (4.13–5.74 folds of raw material) which may reduce the hydration capacity of fibers due to loss of hydrophilic groups.
The ratio of hemicellulose to cellulose dropped markedly from 1.143 to 0.161 in fibers treated at 0 & 5% H2SO4 and these results were in accordance to the previously published reports.7 The gentle decline in cellulose content at higher acid regimes (2.0–5.0%) suggest that minute quantity of cellulose hydrolyzed by concentrated acid regimes that may disrupt crystal region and ultimately reduced the thermal stability.
These results confirmed that acid–alkaline treatments could effectively remove non-cellulosic components and improve some functional properties of IRBF.
Effect of different composition and microstructure on the functional properties of IRBF
The functional properties of fiber would be changed by any treatment, due to changes in fiber components and physical structure.27 Acid–base hydrolysis reduced the starch and cytoplasmic protein contents,3,22 leaving a shell of cell wall structural polysaccharides that may form cavities or spaces. The shell and polysaccharides are responsible for the marked differences in physical properties of IRBF. Along with increment of spaces between fiber particles, these cavities can increase water or oil binding capacity.As shown in Fig. 2a1, the WHC of IRBF produced with 0.2% H2SO4 significantly increased by ∼2-fold (22.45 g g−1). However, when treated with 1.25% H2SO4, the WHC decreased (12.31 g g−1) that was comparable to untreated rice bran sample (10.33 g g−1). The IRBFs with high WHC produced by low acid treatments would be desirable for improving the volume of fecal bulk and preventing constipation.28 Although, the modified fibers possessing low WHC were considered to be undesirable for many food, they still can be potentially used as low calorie bulk ingredients in low moisture food.
Fig. 2a2 showed the WHC is closely related to the hemicelluloses content (R2 = 0.96) at same particle size distributions (250 μm). In this study, the initial acid treatment, 0.2% H2SO4, induced significant increment of WHC, which due to 60% of the starch and almost all of the protein were removed that improving the specific surface area of fiber and the exposure of hydrated hydroxide, carboxyl groups and capillary action of fiber through the spaces between cell wall structures. However, with the increase in acid concentration beyond 1.25% (w/v), the WHC decreased markedly from 12.31 to 8.36 g g−1. The marked reduction in WHC might be due to destruction of integrate fiber matrix and the collapse of the pores as well as the reduction of polar groups, uronic acid groups with the hydrolysis of hemicelluloses.20,29
In contrast to decreased WHC, the OBC (Fig. 2b1) increased (1.87 to 8.69, g g−1) when H2SO4 concentration increased from 0.2% to 3.0%. The high OBC of modified IRBFs suggest that IRBFs have potential to be used as an ingredient in fiber rich foodstuffs requiring oil retention. The increase of OBC linearly correlated to the reduction of starch (R2 = 0.92) and hemicelluloses (R2 = 0.83, Fig. 2b2). With the removal of starch and hemicellulose, hydrophilic groups significantly decreased and left the hydrophobic cell wall as well as a porous structure that increased the capillary attraction of the fiber, and consequently enhanced the oil entrapment and the magnitude of OBC.30 The OBCs for IRBFs were found to be higher than that reported for fibers isolated from some fruits, vegetables and seaweeds (e.g., cauliflower, apple pomace, citrus peel and artichoke, 0.9–2.1 g g−1).31–34 This shows that IRBF has great potential in application in functional foods.
The results for bulk density are illustrated in Fig. 2c1 and c2. It appears that dilute acid (0.2% H2SO4) removed the majority of starch and protein resulting in collapse of rice bran particle or the cell wall shell, and increased the bulk density. After increasing the acid concentration above 0.2%, the bulk density decreased from 0.31 to 0.09 g mL−1. The reason can be explained by the successive removal of remaining starch that leads to greater porosity and smaller particle packing effect of IRBF.6,20 Fig. 2c2 showed that the increment of starch and hemicelluloses contents resulted in the increased bulk density. Especially, the linear fit between hemicelluloses content and bulk density was significant (R2 = 0.93), which was mainly due to the deformation of fiber matrix with the hydrolysis of hemicellulose.
Fig. 2d1 and d2 shows that SWC of modified IRBFs was significantly (P < 0.05) increased (7.01 mL g−1) at 0.2% H2SO4–1.25% KOH regime, but decreased gradually to 2.23 mL g−1 with higher acid regimes. This tremendous reduction in SWC may be associated with decrease of amorphous region which is mainly composed of starch and hemicelluloses. The flexible structure and water affinity of starch and hemicelluloses allowed the fiber matrix to swell. The remaining fiber matrix was composed mainly of dense, crystalline cellulose that does not absorb water and swell. The swelling volume of fiber would be improved by exposing more surface area, polar groups, uronic acid groups and other water binding sites to the surrounding water with the reduction of bulk density.20 However, our results showed that lower bulk density led to lower SWC. Therefore, we suggest that the decreased SWC may be due to reduction of hydration groups on hemicelluloses (R2 = 0.71) and collapse of the flexible regions under higher acid conditions35 and this hypothesis will be examined further by crystallinity results presented below. The weak linear relationship between starch, hemicelluloses contents and SWC suggests that the related factors to SWC of IRBF were more complex.
Fig. 2e1 and e2 shows the values of CEC for native and modified IRBFs. The relative higher CEC (0.214 meq g−1) observed for the sample treated with 0.2% H2SO4 regime compared to untreated material (0.104 meq g−1) probably due to increased exposure of uronic acids by the interruption between cellulose and hemicelluloses linkage. On the other hand, with increasing H2SO4 concentration from 0.6 to 5.0%, the CEC decreased significantly. This may be ascribed to the reduction of carboxyl groups in starch (R2 = 0.79) and hemicelluloses (R2 = 0.99) fractions which act as cation exchangers.30
Scanning electron microscopy (SEM)
The exterior and interior surfaces of IRBFs produced with 0.0, 0.2, 1.25 and 2.0% H2SO4 are shown in Fig. 3. The exterior of the untreated rice bran (Fig. 3a1) had plaster appearance and unique rectangular tiled structure as reported by Watson and Dikeman.36 After treated with 0.2% H2SO4, the ordered tiled surface was changed to more rough as shown in Fig. 3b1. Stronger acid treatment showed deeper degradation of the tiled structure of IRBF. Moreover, we also observed the inner surface of hull that was striated and fibrous in shape as shown in Fig. 3a2. In our case, the inner surface of IRBF produced with 1.25% & 2.0% H2SO4 (Fig. 3c2 & d2) showed a rougher and much more porous texture that proved its decreased bulk density. | ||
| Fig. 3 SEM images of exterior (a1, b1, c1 & d1) and interior (endosperm facing) surface (a2, b2, c2 & d2) of defatted rice bran and IRBF produced with 0.2, 1.25, and 2.0% H2SO4 regimes. | ||
XRD analysis
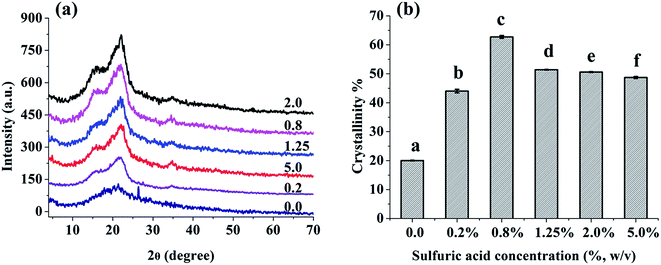
In order to evaluate the changes in crystal structure of IRBF after exposure with acid–base regimes, XRD analysis was performed. The XRD patterns of native and modified IRBF produced with 0.2%, 0.8%, 1.25%, 2.0%, and 5.0% H2SO4 regimes are shown in Fig. 4a. The crystal type of all tested samples showed characteristic of cellulose I crystal form with strong peaks at 2θ = 16° and 22°.37 The crystallinity values (Fig. 4b) of all modified samples were higher than that of untreated rice bran. The crystallinity of IRBF produced with dilute H2SO4 regime (0.2–0.8%) increased by 2–3 folds compared to the raw material. The increase in crystallinity was mainly attributed to the removal of starch and hemicelluloses that formed the amorphous region. The further decline of crystallinity (from 62.7% to 48.7%) of IRBF produced with higher H2SO4 regimes (from 1.25% to 5.0%) was probably due to the disruption of partial crystalline regions of cellulose. It is hypothesized that lower crystallinity might increase the WHC and SWC of fiber, which could increase the transit time of food stuff in the small intestine, and decrease the cholesterol availability in the small intestine.38 Higher crystallinity of fiber would improve the thermal stability of fiber and benefit their applications in reinforcing materials processed at high temperatures.39Thermo gravimetric analysis
IRBFs produced with different acid–base regimes had various thermal stabilities as shown in Fig. 5. The initial minute mass loss below 125 °C for all tested samples was ascribed to the loss of water. The sharp weight loss at ∼300 °C was mainly due to pyrolysis of hemicellulose and cellulose.40 The maximum decomposition temperature for untreated rice bran was 310 °C while that significantly increased to 340–380 °C for IRBF produced with 0.2–0.8% H2SO4 regimes (Fig. 5b). These results are in accordance to the findings of Alemdar and Sain41 who observed that the removal of heat sensitive components (starch, protein & pectin) resulted in higher thermal decomposition temperature of fiber. In our case, the decomposition temperature for IRBF produced with higher H2SO4 regimes (1.25–5.0%, w/v) was almost 350 °C, which was lower than that of sample produced with 0.8% H2SO4 as shown in Fig. 5. The possible reason behind this thermal decomposition variability is the disintegration of crystalline regions of IRBF as the bonds between cellulose chains in crystalline regions are responsible for thermal stability. The current results suggest the reduced crystallinity of IRBF samples (Fig. 4b). Decomposition of lignin in native rice bran was verified by small peak at 412 °C that significantly increased in case of IRBF.40 | ||
| Fig. 5 Thermogravimetric (a) and differential thermogravimetric (b) curves of defatted rice bran before and after modification by a series (0.0, 0.2, 0.8, 1.25, 2.0, 5.0%, w/v) of H2SO4 regimes. | ||
FT-IR spectroscopic analysis
The FT-IR spectra of IRBF samples are illustrated in Fig. 6. The typical band at 1734 cm−1 was responsible for carbonyl group42 in untreated rice bran sample that shifted to longer wave numbers (1737 cm−1) for IRBF samples. The corresponding shift and increase in band intensity suggests the increase in number of free carboxyl groups with the reduction of hydrogen bonds between the acid molecules and cellulose chains42 in the modified IRBFs. | ||
| Fig. 6 FTIR spectra of defatted rice bran before and after modification by a series of (0.0, 0.2, 0.8, 1.25, 2.0, 5.0%, w/v) H2SO4 regimes. | ||
The band at 1654 cm−1 for untreated rice bran sample was related to the stretching of carboxyl groups that are interconnected with cellulose chains by forming intermolecular hydrogen bonds.17 In case of acid–base modified IRBFs, this band shifted to lower wave number (1640 cm−1) and the intensity decreased, which suggests destruction of some of hydrogen bonds between cellulose chains. This destruction was further confirmed by decrease in cellulose content and crystallinity as shown in Fig. 1a and 4b. The disappearance of the band at 1242 cm−1 also suggests a reduction in number of hydrogen bonds in modified IRBFs.42
Conclusions
The current study showed that the physicochemical attributes of cellulosic fraction from defatted rice bran could be enhanced by simple, low cost acid–base method. The dilute acid treatment (0.2–1.25%) increased the WHC two folds. Whereas, higher acid regimes decreased the WHC, bulk density and CEC but increased the OBC and relative crystallinity, which related to the removal of starch and hemicelluloses. The increase in crystallinity improved the thermal stability of modified IRBF as evident from XRD and TGA analysis. The study provides an insight to produce acid–base modified IRBF with improved structural and physicochemical attributes that can satisfy the long lasting wish of food processors to develop intelligent (nutritional & disease prevention) functional foods. Moreover, we provide acid–base standard regimes that can provide thermally suitable IRBF to prepare temperature sensitive processed foods.Acknowledgements
The authors are grateful to Dayang Rice Company of China (Jiangsu, China) for the provision of the rice bran used in this study. This work was financially supported by National 125 Program 2011BAD23B02, 2013AA102207; NSFC 31171686; NSFJiangsu-BK2012556; 111 Project B0702.References
- P. B. Mellen, A. D. Liese, J. A. Tooze, M. Z. Vitolins, L. E. Wagenknecht and D. M. Herrington, Am. J. Clin. Nutr., 2007, 85, 1495–1502 CAS.
- N. M. McKeown, J. B. Meigs, S. Liu, P. W. F. Wilson and P. F. Jacques, Am. J. Clin. Nutr., 2002, 76, 390–398 CAS.
- M. N. Zafar, I. Aslam, R. Nadeem, S. Munir, U. A. Rana and S. U. D. Khan, J. Taiwan Inst. Chem. Eng., 2014, 1–7 Search PubMed.
- B. O. Schneeman, in Dietary fibre and gastrointestinal function, ed. B. V. McCleary and L. Prosky, Blackwell Science, Oxford, 2001, pp. 168–176 Search PubMed.
- P. Gupta and K. S. Premavalli, Int. J. Food Prop., 2011, 14, 397–410 CrossRef CAS PubMed.
- T. Wang, X. H. Sun, J. Raddatz and G. B. Chen, J. Cereal Sci., 2013, 58, 355–361 CrossRef CAS PubMed.
- A. Abdul-Hamid and Y. S. Luan, Food Chem., 2000, 68, 15–19 CrossRef CAS.
- K. Vijayaraghavan and Y. S. Yun, Biotechnol. Adv., 2008, 26, 266–291 CrossRef CAS PubMed.
- C. Bertin, X. Rouau and J. F. Thibault, J. Sci. Food Agric., 1988, 44(1), 15–29 CrossRef CAS PubMed.
- M. B. Bera, Indian Food Ind., 1992, 11, 36–38 Search PubMed.
- R. M. Saunders, Cereal Foods World, 1990, 35, 632–636 Search PubMed.
- J. K. Liu, W. Zhao, H. B. Zhang, Y. Y. Liu and Y. Z. Zhang, Hubei Agr. Sci., 2012, 51(8), 1636–1638 Search PubMed.
- X. H. Xiong, L. P. Zhao, Y. M. Chen, Q. J. Ruan, C. M. Zhang and Y. F. Hua, Food Bioprod. Process., 2015, 94, 239–247 CrossRef CAS PubMed.
- AOAC, Official methods of analysis of AOAC internation, Association of Official Analytical Chemists International, Gaithersburg, MD, 18 edn, 2005 Search PubMed.
- H. Y. Yeh, N. W. Su and M. H. Lee, J. Agric. Food Chem., 2005, 53, 4361–4366 CrossRef CAS PubMed.
- B. G. Unni, A. Borah, S. B. Wann, H. R. Singh, B. Devi and M. Bhattacharjee, Asian J. Exp. Sci., 2009, 23, 103–108 CAS.
- X. F. Sun, R. C. Sun, J. Tomkinson and M. S. Baird, Carbohydr. Polym., 2003, 53, 483–495 CrossRef CAS.
- I. Egüés, C. Sanchez, I. Mondragon and J. Labidi, Bioresour. Technol., 2012, 103, 239–248 CrossRef PubMed.
- A. Sangnark and A. Noomhorm, Food Chem., 2003, 80, 221–229 CrossRef CAS.
- C. F. Chau, Y. T. Wang and Y. L. Wen, Food Chem., 2007, 100, 1402–1408 CrossRef CAS PubMed.
- I. Navarro-González, V. García-Valverde, J. García-Alonso and M. J. Periago, Food Res. Int., 2011, 44, 1528–1535 CrossRef PubMed.
- C. F. Chau and P. C. K. Cheung, Nutr. Res., 1999, 19, 257–265 CrossRef CAS.
- Y. S. Choi, J. H. Choi, D. J. Han, H. Y. Kim, M. A. Lee, H. W. Kim, J. Y. Jeong and C. J. Kim, Meat Sci., 2011, 88(1), 59–66 CrossRef CAS PubMed.
- O. Kanauchi, K. Mitsuyama, Y. Komiyama, M. Yagi, A. Andoh and M. Sata, Int. J. Mol. Med., 2010, 25(4), 547–555 CAS.
- N. Grigelmo-Miguel and O. MartmHn-Belloso, LWT--Food Sci. Technol., 1999, 32, 503–508 CrossRef CAS.
- B. Feng and A. Y. Qiu, Journal of Food and Feed Industry, 1997, 11, 37–39 Search PubMed.
- C. M. Rosell, E. Santos and C. Collar, Food Res. Int., 2009, 42, 176–184 CrossRef CAS PubMed.
- D. Oakenfull, Physiochemical properties of dietary fiber: overview, in Handbook of Dietary Fiber, ed. S. S. Cho and M. L. Dreher, Marcel Dekker, New York, 2001, pp. 195–206 Search PubMed.
- A. Auffret, M. C. Ralet, F. Guillon, J. L. Barry and J. F. Thibault, LWT--Food Sci. Technol., 1994, 27, 166–172 CrossRef CAS.
- C. F. Chau and Y. L. Huang, J. Agric. Food Chem., 2003, 51, 2615–2618 CrossRef CAS PubMed.
- A. Femenia, C. Lefebvre, Y. Thebaudin, J. Robertson and C. Bourgeois, J. Food Sci., 1997, 62, 635–639 CrossRef CAS PubMed.
- F. Figuerola, M. L. Hurtado, A. M. Estévez, I. Chiffelle and F. Asenjo, Food Chem., 2005, 91, 395–401 CrossRef CAS PubMed.
- E. Gómez-Ordoñez, A. Jiménez-Escrig and P. Rupérez, Food Res. Int., 2010, 43, 2289–2294 CrossRef PubMed.
- G. López, G. Ros, F. Rincón, M. J. Periago, M. C. Martinez and J. Ortuño, J. Agric. Food Chem., 1996, 44, 2773–2778 CrossRef.
- S. N. Raghavendra, S. R. Ramachandra Swamy, N. K. Rastogi, K. S. M. S. Raghavarao, S. Kumar and R. N. Tharanathan, J. Food Eng., 2006, 72, 281–286 CrossRef PubMed.
- C. A. Watson and E. Dikeman, Cereal Chem., 1977, 54(1), 120–130 Search PubMed.
- W. S. Chen, H. P. Yu and Y. X. Liu, Carbohydr. Polym., 2011, 61, 1–9 Search PubMed.
- D. T. Gordon, Cereal Foods World, 1989, 34, 517–525 Search PubMed.
- M. Roman and W. T. Winter, Biomacromolecules, 2004, 5, 1671–1677 CrossRef CAS PubMed.
- K. G. Mansaray and A. E. Ghaly, Polym. Degrad. Stab., 1998, 84, 13–21 Search PubMed.
- A. Alemdar and M. Sain, Bioresour. Technol., 2008, 99, 1664–1671 CrossRef CAS PubMed.
- L. Dymińska, M. Szatkowski, M. Wróbel-Kwiatkowska, M. Żuk, A. Kurzawa, W. Syska, A. Gągor, M. Zawadzki, M. Ptak, M. Mączka, J. Hanuza and J. Szopa, J. Biotechnol., 2012, 164, 292–299 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |