Recent advances of curcumin and its analogues in breast cancer prevention and treatment
Abstract
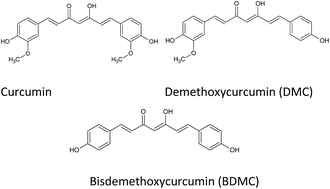
More than 230 000 diagnosed cases of invasive breast cancer in women were estimated in 2014 and an expected 40 000 deaths attributed to the aggressive carcinoma. An effective approach to diminish the morbidity and mortality of breast cancer is the development of chemopreventive and chemotherapeutic agents. Nutraceuticals have demonstrated their ability to proficiently halt carcinogenesis. The administration of natural compounds able to effectively serve as chemoprevention and chemotherapeutics without causing harm or adverse effects is imperative. Curcumin derived from the rhizome of Curcuma longa L., is a common spice of India, used for centuries because of its medicinal properties. The main component of curcumin possesses a wide range of biological activities; anti-proliferative, anti-inflammatory, and apoptotic characteristics modulated through the inactivation of pathways such as EGK and Akt/mTOR. In addition, curcumin alters the expression of cytokines, transcription factors, and enzymes involved in cell vitality. The in vivo application of curcumin in breast cancer is hindered by its limited bioavailabiity. The synthesis of curcumin analogues and delivery via nanoparticles has demonstrated enhanced bioavailability of curcumin in the malignancy. This review focuses on recent developments in the use of curcumin, curcumin analogues, and novel delivery systems as a preventive and therapeutic method for breast cancer.

 Please wait while we load your content...
Please wait while we load your content...