Cs2CO3-promoted [3 + 2] cycloaddition providing an easy protocol toward imidazo[1,2-a]indole derivatives†
Li-Dan Zhang‡
a,
Jiang-Kai Qiu‡ab,
Li-Fang Kongc,
Wen-Juan Hao*a,
Jiao-Na Miaoa,
Shuo Wua,
Bo Jiang*a and
Shu-Jiang Tu*a
aSchool of Chemistry and Chemical Engineering, Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, Jiangsu Normal University, Xuzhou, Jiangsu, P. R. China. E-mail: wjhao@jsnu.edu.cn; jiangchem@jsnu.edu.cn; laotu@jsnu.edu.cn; Fax: +86 51683500065; Tel: +86 51683500065
bBiotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing, 210009, Jiangsu, P. R. China
cDepartment of Basic Teaching, Air Force Logistic Academy, Xuzhou, Jiangsu, P. R. China
First published on 1st September 2015
Abstract
A base-promoted [3 + 2] cycloaddition for the construction of multi-functionalized imidazo[1,2-a]indole derivatives with good yields has been accomplished. This transformation provides an easy and facile protocol for the formation of diverse imidazo[1,2-a]indoles of chemical and biomedical importance using preformed 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles and readily available but-2-ynedioates under mild conditions.
Introduction
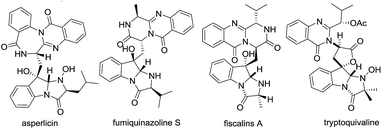
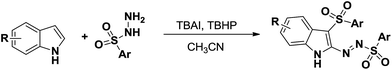
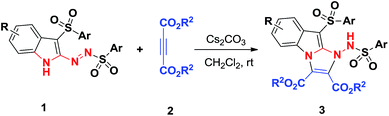
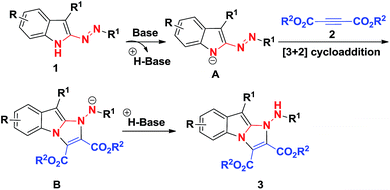
The fused indole nucleus is one of the most important heterocyclic motifs found in natural products, pharmaceutically active compounds and agrochemicals.1 Therefore, the search for an efficient construction of a fused indole skeleton of chemical and biomedical importance is an active but challenging subject in organic synthesis.2 Structurally diverse imidazo[1,2-a]indole subunits commonly exist in nature and are represented by asperlicin,3 fumiquinazoline S,4 fiscalin A,5 and tryptoquivalins6 which exhibit a broad range of biological activities (Fig. 1). For example, asperlicins and its analogues have acted as cholecystokinin antagonist;3 fumiquinazolines have exhibited antifungal activity;4 fiscalins and its analogues were found to serve as the substance P antagonists;5 and tryptoquivalines have shown antibacterial and antibiofilm activity.6 Although these fused imidazo[1,2-a]indoles showed important biological activities, efficient and practical approaches to these molecules have been rarely documented. There are some limited methods for the assembly of imidazo[1,2-a]indoles, which suffer from the tedious steps,7 low efficiency,7,8 and/or the narrow substrate scopes.9 Hence, the exploration of new, facile and versatile method for forming functionalized imidazo[1,2-a]indoles from readily available starting materials is still highly desired but full of challenge.[3 + 2] cycloaddition reactions were one of the key tools that allow the one-step creation of several bonds in a single operation and offer remarkable advantages like convergence, high efficiency, and operational simplicity.10 In recent years, considerable efforts have been devoted to the development of various [3 + 2] cycloaddition reactions toward the formation of various five-membered heterocycles.11 Recently, we reported the unprecedented synthesis of 3-sulfonyl-2-sulfonyldiazenyl-1H-indole derivatives through TBAI/TBHP-mediated oxidative reactions (Scheme 1).12 Considering the nucleophilicity of free N–H of indole ring and the electrophilicity of azo unit, we reasoned that 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles can serve as 1,3-dipoles in the presence of base, allowing [3 + 2] cycloaddition reactions with electron-deficient alkynes for forming important multifunctional imidazo[1,2-a]indoles. Herein we report the successful realization of this concepts via a facile [3 + 2] cycloaddition using preformed 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles and readily available but-2-ynedioates under mild conditions, which providing densely functionalized imidazo[1,2-a]indole-2,3-dicarboxylates in a highly regioselective and functional-group-compatible manner (Scheme 2).
Results and discussion
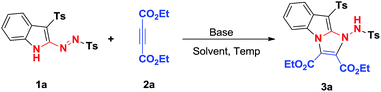
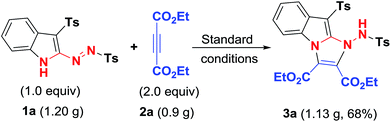
In our previous communication, to explore the potential applications of 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles, [3 + 2] cycloaddition reaction between 3-tosyl-2-(tosyldiazenyl)-1H-indole (1a) and diethyl acetylenedicarboxylate (2a) was carried out in the presence of Et3N in EtOH at room temperature, affording one example of tricyclic pyridazino[3,4-b]indole-3,4-dicarboxylate 3a in 47% yield. After completion of this reaction, we believed that this reaction may be improved by screening appropriate reaction conditions. In order to obtain the optimal reaction conditions, the above model reaction was conducted to investigate the impact of various reaction conditions on the reaction outcome, including solvents, temperatures and bases. The results are listed in Table 1. The reaction using NaOH as a base promoter in EtOH gave a 57% yield of product 3a (entry 2). The use of Cs2CO3 delivered a higher yield (up to 62%, entry 3). The subsequent evaluation of base promoter was performed with EtOH as the reaction solvent. This evaluation showed that other bases including pyridine, K2CO3, and DBU all gave inferior results than that of Cs2CO3 (entries 4–6, respectively, vs. entry 2). Taking Cs2CO3 as the optimized base promoter, the effect of solvent were investigated. The solvent was switched to CH2Cl2, giving access to the expected product 3a in 73% yield (entry 7). Other aprotic solvents, such as acetone, ethyl acetate, acetonitrile, toluene, DMF, DMSO, and 1,4-dioxane, were inferior to CH2Cl2 in terms of reaction yields (entries 8–14). Lowering the amount of Cs2CO3 decreased the yield of product 3a (entry 15). Next, the reaction temperature was evaluated. Raising the temperature slightly to 40 °C proved less efficient, only providing 3a with a 52% yield (entry 16).| Entry | Base (equiv.) | Solvent | t (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (1.0 mmol), base promoter (3.0 equiv.), solvent (2.0 ml), 20 hours.b Isolated yield based on 1.c Not detected (N.D). | ||||
| 1 | Et3N (3.0) | EtOH | 25 | 47 |
| 2 | NaOH (3.0) | EtOH | 25 | 57 |
| 3 | Cs2CO3 (3.0) | EtOH | 25 | 62 |
| 4 | Pyridine (3.0) | EtOH | 25 | 36 |
| 5 | K2CO3 (3.0) | EtOH | 25 | N.Dc |
| 6 | DBU (3.0) | EtOH | 25 | 37 |
| 7 | Cs2CO3 (3.0) | CH2Cl2 | 25 | 73 |
| 8 | Cs2CO3 (3.0) | Acetone | 25 | 37 |
| 9 | Cs2CO3 (3.0) | Ethyl acetate | 25 | 46 |
| 10 | Cs2CO3 (3.0) | CH3CN | 25 | 66 |
| 11 | Cs2CO3 (3.0) | Toluene | 25 | 57 |
| 12 | Cs2CO3 (3.0) | DMF | 25 | 62 |
| 13 | Cs2CO3 (3.0) | DMSO | 25 | N.Dc |
| 14 | Cs2CO3 (3.0) | 1,4-Dioxane | 25 | 56 |
| 15 | Cs2CO3 (2.0) | CH2Cl2 | 25 | 70 |
| 16 | Cs2CO3 (3.0) | CH2Cl2 | 40 | 52 |
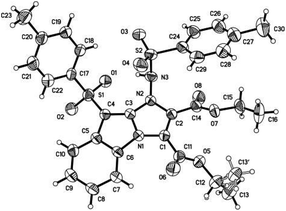
With the optimal conditions to access functional imidazo[1,2-a]indoles in hand, we then turned to evaluating the scope of the Cs2CO3-promoted [3 + 2] cycloaddition reaction for different substituted 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles and acetylenedicarboxylates (Scheme 3). Upon repeating the reaction with diethyl acetylenedicarboxylate 2a, we are pleased to find that a variety of functional groups of 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles including bearing electron-rich, electron-neutral, and electron-poor groups were well-tolerated under the above conditions to give corresponding adducts 3b–3g in good yields of 56–78%. Generally, substituents on the benzenesulfonyl nucleus bearing withdrawing groups showed the lower reactivity than their electron-donating counterparts. For instance, 1c with methoxy group was treated with diethyl but-2-ynedioate 2a, affording the corresponding product 3c in a 78% yield. In contrast, the presence of a bromo group of substrate 1g resulted in a 56% chemical yield. These observations indicated that electronic nature of substituents on the arylsulfonyl moiety had some effect on the reaction efficiency. Alternatively, dimethyl but-2-ynedioate 3b was successfully engaged in this [3 + 2] cycloaddition reaction, and the corresponding imidazo[1,2-a]indole products 3h–3l were obtained with 60–82% yields. In view of these results, we considered varying the substituents of the indole ring and select 5-methoxyl counterpart as a representative substrate to explore the feasibility of the reaction. As we had expected, 5-methoxylindoles bearing electron-donating, -neutral, or -withdrawing groups on the arylsulfonyl motif were reacted with acetylenedicarboxylates 2a and 2b, participating in the current Cs2CO3-promoted [3 + 2] cycloaddition successfully, and the desired imidazo[1,2-a]indole 3m–3q were formed with higher yields. Although imidazo[1,2-a]indoles 3 were characterized by their NMR spectroscopy and HRMS, their structures were determined by X-ray diffraction of compound 3a (Fig. 2) (see the ESI†).
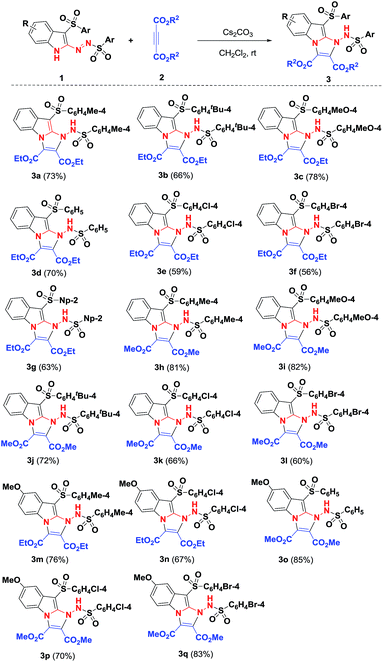 | ||
| Scheme 3 [3 + 2] cycloaddition reactions for forming products 3. Reaction conditions: 1 (0.5 mmol), 2 (1.0 mmol), Cs2CO3 (3.0 equiv.) were stirred in CH2Cl2 (2.0 ml) at room temperature for 20 h. | ||
To develop the efficiency of our method, a gram-scale reaction of easily prepared 1a with 2a was carried out under the standard conditions, providing in a 68% yield of 3a (Scheme 4), which offered the potential application in organic synthesis.
On the basis of our experimental observations and literature precedents,11 a plausible mechanistic pathway to products 3 is illustrated in Scheme 5. Initially, in the presence of base, indoles 1 undergoes deprotonation to indol-1-ide intermediate A, followed by [3 + 2] cycloaddition with acetylenedicarboxylates 2 to yield imidazo[1,2-a]indole intermediate B, which converts into the final products 3 via protonation.
Conclusions
In conclusion, a reliable and mild base-promoted [3 + 2] cycloaddition reaction has been developed using readily preformed 3-sulfonyl-2-sulfonyldiazenyl-1H-indoles and acetylenedicarboxylates, by which a wide set of imidazo[1,2-a]indole derivatives with a wide diversity in substituents are rapidly synthesized in a convergent fashion. This straightforward and operationally simple method allows installing two C–N bonds and imidazole skeleton. The flexibility of structural modification and mild reaction conditions make this strategy highly viable for future applications.Experimental section
General
White solid, mp 185–186 °C; IR: 3134 (NH), 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1613 (C
O), 1613 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 8.01–7.88 (m, 4H), 7.60 (d, J = 8.4 Hz, 2H), 7.40–7.37 (m, 3H), 7.31 (d, J = 8.4 Hz, 2H), 7.27–7.23 (m, 1H), 4.45–4.39 (m, 2H), 3.89–3.68 (m, 2H), 2.38 (s, 3H), 2.32 (s, 3H), 1.29 (t, J = 7.2 Hz, 3H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.6, 156.7, 143.2, 141.7, 140.4, 130.3, 129.9, 129.8, 128.3, 126.9, 125.6, 124.9, 121.5, 119.5, 114.2, 90.3, 63.4, 62.8, 21.5, 21.4, 14.1, 13.8. HRMS (ESI) m/z: calcd for C30H29N3O8S2Na: 646.1288 [M + Na]+; found: 646.1299.
O). 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 8.01–7.88 (m, 4H), 7.60 (d, J = 8.4 Hz, 2H), 7.40–7.37 (m, 3H), 7.31 (d, J = 8.4 Hz, 2H), 7.27–7.23 (m, 1H), 4.45–4.39 (m, 2H), 3.89–3.68 (m, 2H), 2.38 (s, 3H), 2.32 (s, 3H), 1.29 (t, J = 7.2 Hz, 3H), 1.15 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.6, 156.7, 143.2, 141.7, 140.4, 130.3, 129.9, 129.8, 128.3, 126.9, 125.6, 124.9, 121.5, 119.5, 114.2, 90.3, 63.4, 62.8, 21.5, 21.4, 14.1, 13.8. HRMS (ESI) m/z: calcd for C30H29N3O8S2Na: 646.1288 [M + Na]+; found: 646.1299.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1612 (C
O), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 10.12 (s, 1H), 8.20 (d, J = 8.4 Hz, 1H), 7.82–7.75 (m, 2H), 7.69–7.59 (m, 3H), 7.42–7.36 (m, 2H), 7.29 (s, 1H), 7.28–7.23 (m, 2H), 7.21–7.15 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.45 (q, J = 7.2 Hz, 2H), 1.48 (t, J = 6.8 Hz, 3H), 1.45 (t, J = 6.8 Hz, 3H), 1.26 (s, 9H), 1.08 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 159.0, 158.7, 157.0, 156.3, 140.3, 138.0, 131.4, 129.5, 129.1, 128.7, 126.5, 125.9, 124.5, 121.5, 118.8, 116.2, 114.3, 88.9, 62.8, 35.1, 31.0, 30.6, 14.0, 14.0. HRMS (ESI, m/z): calculated for C36H40N3O8S2 [M − H]− 706.2251, found 706.2252.
O). 1H NMR (400 MHz, CDCl3) δ 10.12 (s, 1H), 8.20 (d, J = 8.4 Hz, 1H), 7.82–7.75 (m, 2H), 7.69–7.59 (m, 3H), 7.42–7.36 (m, 2H), 7.29 (s, 1H), 7.28–7.23 (m, 2H), 7.21–7.15 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.45 (q, J = 7.2 Hz, 2H), 1.48 (t, J = 6.8 Hz, 3H), 1.45 (t, J = 6.8 Hz, 3H), 1.26 (s, 9H), 1.08 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 159.0, 158.7, 157.0, 156.3, 140.3, 138.0, 131.4, 129.5, 129.1, 128.7, 126.5, 125.9, 124.5, 121.5, 118.8, 116.2, 114.3, 88.9, 62.8, 35.1, 31.0, 30.6, 14.0, 14.0. HRMS (ESI, m/z): calculated for C36H40N3O8S2 [M − H]− 706.2251, found 706.2252.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1612 (C
O), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.85–7.82 (m, 2H), 7.66–7.62 (m, 3H), 6.92–6.84 (m, 2H), 6.82–6.73 (m, 2H), 4.51 (q, J = 7.2 Hz, 2H), 4.40 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 3.66 (s, 3H), 1.47–1.43 (m, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.8, 162.7, 158.7, 156.8, 140.2, 136.4, 130.6, 129.9, 129.1, 125.6, 124.8, 121.4, 119.4, 115.0, 114.5, 114.2, 90.6, 63.3, 62.8, 56.2, 56.0, 14.1, 13.8. HRMS (ESI, m/z): calculated for C30H28N3O10S2 [M − H]− 654.1215, found 654.1211.
O). 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.85–7.82 (m, 2H), 7.66–7.62 (m, 3H), 6.92–6.84 (m, 2H), 6.82–6.73 (m, 2H), 4.51 (q, J = 7.2 Hz, 2H), 4.40 (q, J = 7.2 Hz, 2H), 3.80 (s, 3H), 3.66 (s, 3H), 1.47–1.43 (m, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.8, 162.7, 158.7, 156.8, 140.2, 136.4, 130.6, 129.9, 129.1, 125.6, 124.8, 121.4, 119.4, 115.0, 114.5, 114.2, 90.6, 63.3, 62.8, 56.2, 56.0, 14.1, 13.8. HRMS (ESI, m/z): calculated for C30H28N3O10S2 [M − H]− 654.1215, found 654.1211.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.97–7.90 (m, 2H), 7.76–7.73 (m, 2H), 7.68 (d, J = 8.0 Hz, 1H), 7.55–7.47 (m, 2H), 7.41–7.38 (m, 4H), 7.34–7.30 (m, 1H), 7.26–7.19 (m, 1H), 4.50 (q, J = 7.2 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 1.43 (q, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 158.5, 156.9, 143.1, 138.5, 135.7, 134.6, 132.6, 129.4, 128.8(9), 128.8(5), 126.7, 126.3, 124.8, 121.7, 118.7, 116.2, 114.5, 62.9, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H24N3O8S2 [M − H]− 594.0999, found 594.1021.
O). 1H NMR (400 MHz, CDCl3) δ 9.93 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.97–7.90 (m, 2H), 7.76–7.73 (m, 2H), 7.68 (d, J = 8.0 Hz, 1H), 7.55–7.47 (m, 2H), 7.41–7.38 (m, 4H), 7.34–7.30 (m, 1H), 7.26–7.19 (m, 1H), 4.50 (q, J = 7.2 Hz, 2H), 4.32 (q, J = 7.2 Hz, 2H), 1.43 (q, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 158.5, 156.9, 143.1, 138.5, 135.7, 134.6, 132.6, 129.4, 128.8(9), 128.8(5), 126.7, 126.3, 124.8, 121.7, 118.7, 116.2, 114.5, 62.9, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H24N3O8S2 [M − H]− 594.0999, found 594.1021.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 (C
O), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.92–7.86 (m, 2H), 7.72 (m, 1H), 7.70–7.63 (m, 2H), 7.43–7.41 (m, 1H), 7.41–7.39 (m, 1H), 7.36–7.32 (m, 3H), 7.28–7.21 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.29 (q, J = 7.2 Hz, 2H), 1.46–1.42 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 158.4, 156.8, 141.7, 139.1, 138.7, 134.2, 130.3, 129.8, 129.2, 129.1, 127.8, 126.7, 125.1, 122.1, 118.7, 116.5, 114.5, 88.4, 63.1, 63.0, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H22Cl2N3O8S2 [M − H]− 662.0220, found 662.0225.
O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.92–7.86 (m, 2H), 7.72 (m, 1H), 7.70–7.63 (m, 2H), 7.43–7.41 (m, 1H), 7.41–7.39 (m, 1H), 7.36–7.32 (m, 3H), 7.28–7.21 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.29 (q, J = 7.2 Hz, 2H), 1.46–1.42 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 158.4, 156.8, 141.7, 139.1, 138.7, 134.2, 130.3, 129.8, 129.2, 129.1, 127.8, 126.7, 125.1, 122.1, 118.7, 116.5, 114.5, 88.4, 63.1, 63.0, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H22Cl2N3O8S2 [M − H]− 662.0220, found 662.0225.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 (C
O), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.82–7.80 (m, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.63–7.54 (m, 4H), 7.56–7.47 (m, 2H), 7.37–7.35 (m, 1H), 7.28–7.24 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.29 (q, J = 7.2 Hz, 2H), 1.45–1.42 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 158.4, 156.8, 142.2, 138.7, 134.8, 132.8, 132.2, 130.3, 129.3, 129.0, 127.7, 126.7, 125.1, 122.1, 118.8, 116.6, 114.5, 88.3, 63.1, 63.0, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H22Br2N3O8S2 [M − H]− 751.9190, found 751.9193.
O). 1H NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.82–7.80 (m, 2H), 7.73 (d, J = 8.0 Hz, 1H), 7.63–7.54 (m, 4H), 7.56–7.47 (m, 2H), 7.37–7.35 (m, 1H), 7.28–7.24 (m, 1H), 4.51 (q, J = 7.2 Hz, 2H), 4.29 (q, J = 7.2 Hz, 2H), 1.45–1.42 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 158.4, 156.8, 142.2, 138.7, 134.8, 132.8, 132.2, 130.3, 129.3, 129.0, 127.7, 126.7, 125.1, 122.1, 118.8, 116.6, 114.5, 88.3, 63.1, 63.0, 14.0, 14.0. HRMS (ESI, m/z): calculated for C28H22Br2N3O8S2 [M − H]− 751.9190, found 751.9193.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611 (C
O), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, DMSO-d6) δ 12.39 (s, 1H), 8.81 (s, 1H), 8.31 (s, 1H), 8.11–8.05 (m, 6H), 8.00–7.94 (m, 3H), 7.75–7.73 (m, 2H), 7.70–7.55 (m, 3H), 7.42–7.38 (m, 1H), 7.26–7.24 (m, 1H), 4.36–4.34 (m, 2H), 3.57 (s, 1H), 3.00 (s, 1H), 1.22 (t, J = 7.2 Hz, 3H), 0.65 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.6, 156.5, 141.3, 140.9, 135.1, 134.7, 132.1, 132.0, 130.0, 129.4, 129.0, 128.2, 127.7, 125.6, 125.1, 123.2, 122.6, 121.6, 119.6, 114.1, 90.0, 63.4, 62.5, 14.1, 13.1. HRMS (ESI, m/z): calculated for C36H28N3O8S2 [M − H]− 694.1312, found 684.1309.
O). 1H NMR (400 MHz, DMSO-d6) δ 12.39 (s, 1H), 8.81 (s, 1H), 8.31 (s, 1H), 8.11–8.05 (m, 6H), 8.00–7.94 (m, 3H), 7.75–7.73 (m, 2H), 7.70–7.55 (m, 3H), 7.42–7.38 (m, 1H), 7.26–7.24 (m, 1H), 4.36–4.34 (m, 2H), 3.57 (s, 1H), 3.00 (s, 1H), 1.22 (t, J = 7.2 Hz, 3H), 0.65 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.6, 156.5, 141.3, 140.9, 135.1, 134.7, 132.1, 132.0, 130.0, 129.4, 129.0, 128.2, 127.7, 125.6, 125.1, 123.2, 122.6, 121.6, 119.6, 114.1, 90.0, 63.4, 62.5, 14.1, 13.1. HRMS (ESI, m/z): calculated for C36H28N3O8S2 [M − H]− 694.1312, found 684.1309.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1616 (C
O), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.95 (s, 1H), 8.21–8.18 (m, 1H), 7.82–7.80 (m, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.61–7.57 (m, 2H), 7.33–7.30 (m, 1H), 7.26–7.18 (m, 3H), 7.13 (d, J = 8.0 Hz, 2H), 4.04 (s, 3H), 3.90 (s, 3H), 2.35 (s, 3H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.0, 157.4, 146.2, 143.5, 140.3, 138.4, 132.1, 130.1(09), 130.1(07), 129.5, 129.4, 129.3, 128.9, 126.6, 126.3, 124.7, 121.7, 118.7, 116.0, 114.4, 89.1, 53.4, 53.3, 21.5. HRMS (ESI, m/z): calculated for C28H24N3O8S2 [M − H]− 594.0999, found 594.0999.
O). 1H NMR (400 MHz, CDCl3) δ 9.95 (s, 1H), 8.21–8.18 (m, 1H), 7.82–7.80 (m, 2H), 7.71 (d, J = 8.0 Hz, 1H), 7.61–7.57 (m, 2H), 7.33–7.30 (m, 1H), 7.26–7.18 (m, 3H), 7.13 (d, J = 8.0 Hz, 2H), 4.04 (s, 3H), 3.90 (s, 3H), 2.35 (s, 3H), 2.24 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 159.0, 157.4, 146.2, 143.5, 140.3, 138.4, 132.1, 130.1(09), 130.1(07), 129.5, 129.4, 129.3, 128.9, 126.6, 126.3, 124.7, 121.7, 118.7, 116.0, 114.4, 89.1, 53.4, 53.3, 21.5. HRMS (ESI, m/z): calculated for C28H24N3O8S2 [M − H]− 594.0999, found 594.0999.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611 (C
O), 1611 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.94 (s, 1H), 8.22 (d, J = 8.4 Hz, 1H), 7.91–7.83 (m, 2H), 7.71–7.56 (m, 3H), 7.33–7.29 (m, 1H), 7.25–7.19 (m, 1H), 6.92–6.85 (m, 2H), 6.82–6.72 (m, 2H), 4.04 (s, 3H), 3.93 (s, 3H), 3.80 (s, 3H), 3.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.7, 162.9, 159.0, 157.5, 138.3, 134.9, 131.2, 129.4, 128.5, 126.6, 126.1, 124.7, 121.6, 118.6, 116.0, 114.8, 114.4, 114.1, 89.5, 55.7, 55.5, 53.4, 53.2. HRMS (ESI, m/z): calculated for C28H24N3O10S2 [M − H]− 626.0897, found 626.0897.
O). 1H NMR (400 MHz, CDCl3) δ 9.94 (s, 1H), 8.22 (d, J = 8.4 Hz, 1H), 7.91–7.83 (m, 2H), 7.71–7.56 (m, 3H), 7.33–7.29 (m, 1H), 7.25–7.19 (m, 1H), 6.92–6.85 (m, 2H), 6.82–6.72 (m, 2H), 4.04 (s, 3H), 3.93 (s, 3H), 3.80 (s, 3H), 3.67 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.7, 162.9, 159.0, 157.5, 138.3, 134.9, 131.2, 129.4, 128.5, 126.6, 126.1, 124.7, 121.6, 118.6, 116.0, 114.8, 114.4, 114.1, 89.5, 55.7, 55.5, 53.4, 53.2. HRMS (ESI, m/z): calculated for C28H24N3O10S2 [M − H]− 626.0897, found 626.0897.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614 (C
O), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 10.12 (s, 1H), 8.18 (d, J = 8.0 Hz, 1H), 7.81–7.76 (m, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.63–7.59 (m, 2H), 7.40–7.37 (m, 2H), 7.30 (s, 3H), 7.19–7.17 (m, 1H), 4.05 (s, 3H), 3.98 (s, 3H), 1.26 (s, 9H), 1.09 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 159.0, 157.5, 156.4, 140.2, 137.9, 131.4, 130.9, 129.3, 129.1, 128.8, 128.7, 126.5, 126.0, 125.9, 124.6, 121.6, 118.8, 116.1, 114.3, 89.2, 53.4, 53.3, 35.1(2), 35.1(06), 31.0, 30.7. HRMS (ESI, m/z): calculated for C34H36N3O8S2 [M − H]− 678.1938, found 678.1947.
O). 1H NMR (400 MHz, CDCl3) δ 10.12 (s, 1H), 8.18 (d, J = 8.0 Hz, 1H), 7.81–7.76 (m, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.63–7.59 (m, 2H), 7.40–7.37 (m, 2H), 7.30 (s, 3H), 7.19–7.17 (m, 1H), 4.05 (s, 3H), 3.98 (s, 3H), 1.26 (s, 9H), 1.09 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 159.0, 157.5, 156.4, 140.2, 137.9, 131.4, 130.9, 129.3, 129.1, 128.8, 128.7, 126.5, 126.0, 125.9, 124.6, 121.6, 118.8, 116.1, 114.3, 89.2, 53.4, 53.3, 35.1(2), 35.1(06), 31.0, 30.7. HRMS (ESI, m/z): calculated for C34H36N3O8S2 [M − H]− 678.1938, found 678.1947.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 (C
O), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.96–7.90 (m, 2H), 7.75 (d, J = 8.0 Hz, 1H), 7.71–7.65 (m, 2H), 7.46–7.34 (m, 5H), 7.30–7.28 (m, 1H), 4.04 (s, 3H), 3.83 (s, 3H). HRMS (ESI, m/z): calculated for C26H18Cl2N3O8S2 [M − H]− 633.9907, found 633.9885.
O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.96–7.90 (m, 2H), 7.75 (d, J = 8.0 Hz, 1H), 7.71–7.65 (m, 2H), 7.46–7.34 (m, 5H), 7.30–7.28 (m, 1H), 4.04 (s, 3H), 3.83 (s, 3H). HRMS (ESI, m/z): calculated for C26H18Cl2N3O8S2 [M − H]− 633.9907, found 633.9885.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.95–7.87 (m, 2H), 7.75 (d, J = 8.0 Hz, 1H), 7.71–7.62 (m, 2H), 7.48–7.33 (m, 5H), 4.04 (s, 3H), 3.83 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.2, 159.0, 145.9, 145.6, 141.2, 134.7, 131.9, 131.2, 130.8, 129.4, 129.3, 125.8, 125.5, 124.2, 123.5, 119.8, 119.1, 114.7, 110.6, 86.7, 53.1, 53.1. HRMS (ESI, m/z): calculated for C26H18Br2N3O8S2 [M − H]− 721.8896, found 721.8891.
O). 1H NMR (400 MHz, CDCl3) δ 9.70 (s, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.95–7.87 (m, 2H), 7.75 (d, J = 8.0 Hz, 1H), 7.71–7.62 (m, 2H), 7.48–7.33 (m, 5H), 4.04 (s, 3H), 3.83 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.2, 159.0, 145.9, 145.6, 141.2, 134.7, 131.9, 131.2, 130.8, 129.4, 129.3, 125.8, 125.5, 124.2, 123.5, 119.8, 119.1, 114.7, 110.6, 86.7, 53.1, 53.1. HRMS (ESI, m/z): calculated for C26H18Br2N3O8S2 [M − H]− 721.8896, found 721.8891.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1610 (C
O), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.92 (s, 1H), 8.13 (m, 1H), 7.77–7.73 (m, 2H), 7.60–7.58 (m, 2H), 7.20–7.18 (m, 2H), 7.12–7.09 (m, 3H), 6.79–6.77 (m, 1H), 4.48 (q, J = 7.2 Hz, 2H), 4.35 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H), 2.34 (s, 3H), 2.26 (s, 3H), 1.43 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 158.6, 157.2, 157.0, 146.0, 143.4, 140.4, 138.4, 132.2, 129.5, 128.9, 126.1, 121.4, 116.0, 115.5, 110.8, 100.7, 88.6, 62.9, 62.7, 55.7, 21.6, 21.5, 14.0, 14.0. HRMS (ESI, m/z): calculated for C31H30N3O9S2 [M − H]− 652.1418, found 652.1438.
O). 1H NMR (400 MHz, CDCl3) δ 9.92 (s, 1H), 8.13 (m, 1H), 7.77–7.73 (m, 2H), 7.60–7.58 (m, 2H), 7.20–7.18 (m, 2H), 7.12–7.09 (m, 3H), 6.79–6.77 (m, 1H), 4.48 (q, J = 7.2 Hz, 2H), 4.35 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H), 2.34 (s, 3H), 2.26 (s, 3H), 1.43 (t, J = 7.2 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 158.6, 157.2, 157.0, 146.0, 143.4, 140.4, 138.4, 132.2, 129.5, 128.9, 126.1, 121.4, 116.0, 115.5, 110.8, 100.7, 88.6, 62.9, 62.7, 55.7, 21.6, 21.5, 14.0, 14.0. HRMS (ESI, m/z): calculated for C31H30N3O9S2 [M − H]− 652.1418, found 652.1438.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614 (C
O), 1614 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (m, 2H), 8.04–8.00 (m, 2H), 7.86–7.83 (m, 1H), 7.50–7.45 (m, 2H), 7.16–7.12 (m, 2H), 6.33–6.31 (m, 1H), 5.92–5.90 (m, 1H), 4.30 (q, J = 7.2, 2H), 3.96 (m, 1H), 3.70–3.59 (m, 1H), 3.37 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H), 1.14 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.9, 158.6, 156.7, 145.7, 145.4, 141.3, 136.4, 134.5, 134.1, 132.1, 129.2, 129.1, 129.0, 128.2, 120.7, 115.6, 110.6, 108.4, 86.7, 62.2, 61.9, 55.7, 14.3, 13.9. HRMS (ESI, m/z): calculated for C29H24Cl2N3O9S2 [M − H]− 692.0325, found 692.0313.
O). 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (m, 2H), 8.04–8.00 (m, 2H), 7.86–7.83 (m, 1H), 7.50–7.45 (m, 2H), 7.16–7.12 (m, 2H), 6.33–6.31 (m, 1H), 5.92–5.90 (m, 1H), 4.30 (q, J = 7.2, 2H), 3.96 (m, 1H), 3.70–3.59 (m, 1H), 3.37 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H), 1.14 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 158.9, 158.6, 156.7, 145.7, 145.4, 141.3, 136.4, 134.5, 134.1, 132.1, 129.2, 129.1, 129.0, 128.2, 120.7, 115.6, 110.6, 108.4, 86.7, 62.2, 61.9, 55.7, 14.3, 13.9. HRMS (ESI, m/z): calculated for C29H24Cl2N3O9S2 [M − H]− 692.0325, found 692.0313.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 8.13–8.10 (m, 1H), 7.94–7.91 (m, 2H), 7.75–7.71 (m, 2H), 7.57–7.47 (m, 2H), 7.47–7.37 (m, 4H), 7.12–7.10 (m, 1H), 6.81–6.78 (m, 1H), 4.01 (s, 3H), 3.84 (s, 3H), 3.82 (s, 3H). HRMS (ESI, m/z): calculated for C27H22N3O9S2 [M − H]− 596.0792, found 596.0793.
O). 1H NMR (400 MHz, CDCl3) δ 8.13–8.10 (m, 1H), 7.94–7.91 (m, 2H), 7.75–7.71 (m, 2H), 7.57–7.47 (m, 2H), 7.47–7.37 (m, 4H), 7.12–7.10 (m, 1H), 6.81–6.78 (m, 1H), 4.01 (s, 3H), 3.84 (s, 3H), 3.82 (s, 3H). HRMS (ESI, m/z): calculated for C27H22N3O9S2 [M − H]− 596.0792, found 596.0793.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.8 Hz, 2H), 8.03–8.01 (m, 2H), 7.84 (d, J = 9.2 Hz, 1H), 7.49–7.45 (m, 2H), 7.18–7.14 (m, 2H), 6.34–6.31 (m, 1H), 5.91 (d, J = 2.4 Hz, 1H), 3.42 (s, 3H), 3.35 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.3, 159.0, 156.7, 145.3, 141.2, 136.5, 132.0, 129.2, 129.1, 129.0, 128.2, 120.7, 115.5, 108.5, 101.8, 86.8, 55.7, 53.0, 53.0. HRMS (ESI, m/z): calculated for C27H20Cl2N3O9S2 [M − H]+ 664.0012, found 664.0059.
O). 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.8 Hz, 2H), 8.03–8.01 (m, 2H), 7.84 (d, J = 9.2 Hz, 1H), 7.49–7.45 (m, 2H), 7.18–7.14 (m, 2H), 6.34–6.31 (m, 1H), 5.91 (d, J = 2.4 Hz, 1H), 3.42 (s, 3H), 3.35 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 159.3, 159.0, 156.7, 145.3, 141.2, 136.5, 132.0, 129.2, 129.1, 129.0, 128.2, 120.7, 115.5, 108.5, 101.8, 86.8, 55.7, 53.0, 53.0. HRMS (ESI, m/z): calculated for C27H20Cl2N3O9S2 [M − H]+ 664.0012, found 664.0059.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1616 (C
O), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 8.13 (d, J = 9.2 Hz, 1H), 7.94–7.91 (m, 2H), 7.75–7.73 (m, 2H), 7.57–7.48 (m, 2H), 7.48–7.35–7.31 (m, 4H), 7.12–7.10 (m, 1H), 6.82–6.79 (m, 1H), 4.02 (s, 3H), 3.85 (s, 3H), 3.83 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.9, 157.4, 143.1, 138.5, 135.7, 134.6, 132.7, 130.7, 130.1, 129.4, 128.9, 128.6, 126.2, 121.3, 115.9, 115.5, 111.1, 100.9, 88.6, 55.6, 53.4, 53.2. HRMS (ESI, m/z): calculated for C27H20Br2N3O9S2 [M − H]−, 751.9008, found 751.9023.
O). 1H NMR (400 MHz, CDCl3) δ 9.91 (s, 1H), 8.13 (d, J = 9.2 Hz, 1H), 7.94–7.91 (m, 2H), 7.75–7.73 (m, 2H), 7.57–7.48 (m, 2H), 7.48–7.35–7.31 (m, 4H), 7.12–7.10 (m, 1H), 6.82–6.79 (m, 1H), 4.02 (s, 3H), 3.85 (s, 3H), 3.83 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 158.9, 157.4, 143.1, 138.5, 135.7, 134.6, 132.7, 130.7, 130.1, 129.4, 128.9, 128.6, 126.2, 121.3, 115.9, 115.5, 111.1, 100.9, 88.6, 55.6, 53.4, 53.2. HRMS (ESI, m/z): calculated for C27H20Br2N3O9S2 [M − H]−, 751.9008, found 751.9023.Acknowledgements
We are grateful for financial support from the NSFC (No. 21232004, and 21472071), PAPD of Jiangsu Higher Education Institutions, the Qing Lan Project (12QLG006), and the Outstanding Youth Fund of Jiangsu Normal University (YQ2015003), and NSF of Jiangsu Normal University (14XLR005), the Open Foundation of Jiangsu Key Laboratory (K201505) and National Students' Innovative Training Program (grant number 201410320018).Notes and references
- (a) H.-J. Knölker and K. R. Reddy, Chem. Rev., 2002, 102, 4303 CrossRef PubMed; (b) R. A. Jones, in Comprehensive Heterocyclic Chemistry, ed. R. A. Katritzky, W. Reese, Pergamon, New York, 1984, vol. 4, p. 201 Search PubMed; (c) R. J. Sundberg, in The Chemistry of Indoles, ed. A. T. Blomquist, Academic, New York, 1970 Search PubMed; (d) W. A. Remers, in The Chemistry of Heterocyclic Compounds, ed. W. J. Houlihan, Wiley-Interscience, New York, 1973, vol. 25, Part I, ch. 1 Search PubMed; (e) S. Hibino and T. Choshi, Nat. Prod. Rep., 2001, 18, 66 RSC; (f) Y. Nishizawa, Nature, 1984, 308, 693 CrossRef PubMed; (g) Y. Nishizawa, Nature, 1988, 334, 661 CrossRef PubMed; (h) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (i) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed.
- (a) M. Platon, R. Amardeil, L. Djakovitch and J.-C. Hierso, Chem. Soc. Rev., 2012, 41, 3929 RSC; (b) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed; (c) R. Vicente, Org. Biomol. Chem., 2011, 9, 6469 RSC; (d) K. Sapeta, T. P. Lebold and M. A. Kerr, Synlett, 2011, 1495 CAS; (e) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC.
- R. S. Chang, V. J. Lotti, R. L. Monaghan, J. Birnbaum, E. O. Stapley, M. A. Goetz, G. Albers-Schonberg, A. A. Patchett, J. M. Liesch, O. D. Hensens and J. P. Springer, Science, 1985, 230, 177 CAS.
- G. N. Belofsky, M. Anguera, P. R. Jensen, W. Fenical and M. Kock, Chem.–Eur. J., 2000, 6, 1355 CrossRef CAS.
- S. M. Wong, L. L. Musza, G. C. Kydd, R. Kullnig, A. M. Gillum and R. Cooper, J. Antibiot., 1993, 46, 545 CrossRef CAS.
- N. M. Gomes, L. J. Bessa, S. Buttachon, P. M. Costa, A. Kijjoa, J. Buaruang, T. Dethoup, A. M. S. Silva and A. Kijjoa, Mar. Drugs, 2014, 12, 822 CrossRef PubMed.
- (a) P. M. J. Lory, R. C. F. Jones, J. N. Iley, S. J. Coles and M. B. Hursthouse, Org. Biomol. Chem., 2006, 4, 3155 RSC; (b) T. Takatsu, Niigata Yakka Daigaku Kenkyu Hokoku, 1994, 14, 1 CAS.
- M. S. Mishina, A. Yu. Ivanov, D. V. Dar'in and P. S. Lobanov, Chem. Heterocycl. Compd., 2013, 49, 648 CrossRef CAS.
- (a) X. Shang, C. Chen, H. Qiu and W. Chen, Tetrahedron, 2014, 70, 3073 CrossRef CAS PubMed; (b) I. T. Forbes, H. K. A. Morgan and M. Thompson, Synth. Commun., 1996, 26, 745 CrossRef CAS PubMed; (c) M. A. Khan, J. B. Polya and B. M. Lynch, Can. J. Chem., 1968, 46, 2629 CrossRef CAS.
- (a) J.-D. Li, H.-B. Zhao, X.-J. Jiang, X.-C. Wang, H.-M. Hu, L. Yu and Y.-D. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6306 CrossRef CAS PubMed; (b) J. Wu, C. Tang, L. Chen, Y. Qiao, M. Geng and Y. Ye, Org. Lett., 2015, 17, 1656 CrossRef CAS PubMed; (c) A. K. Pandey, A. Ghosh and P. Banerjee, Eur. J. Org. Chem., 2015, 2517 CrossRef PubMed; (d) R. Long, J. Huang, W. Shao, S. Liu, Y. Lan, J. Gong and Z. Yang, Nat. Commun., 2014, 5, 5707 CrossRef CAS PubMed; (e) K. Sugimoto, N. Yamamoto, D. Tominaga and Y. Matsuya, Org. Lett., 2015, 17, 1320 CrossRef CAS PubMed; (f) C. Xu, B. Zheng, J. Suo, C. Ding and X. Hou, Angew. Chem., Int. Ed., 2015, 54, 1604 CrossRef CAS PubMed; (g) T. Arai, H. Ogawa, A. Awata, M. Sato, M. Watabe and M. Yamanaka, Angew. Chem., Int. Ed., 2015, 54, 1595 CrossRef CAS PubMed.
- (a) D. Chen, C. Timmons, H.-X. Wei and G. Li, J. Org. Chem., 2003, 68, 5742 CrossRef CAS PubMed; (b) H.-X. Wei, S. H. Kim and G. Li, J. Org. Chem., 2002, 67, 4777 CrossRef CAS PubMed; (c) G. Li, H.-X. Wei, S. H. Kim and M. D. Carducci, Angew. Chem., Int. Ed., 2001, 40, 4277 CrossRef CAS; (d) J. Zhang, Z. Chen, H.-H. Wu and J. Zhang, Chem. Commun., 2012, 48, 1817 RSC; (e) Z. Chen, Z. Tian, J. Zhang, J. Ma and J. Zhang, Chem.–Eur. J., 2012, 18, 8591 CrossRef CAS PubMed; (f) Z. Chen, L. Wei and J. Zhang, Org. Lett., 1170, 20(11), 13 Search PubMed; (g) R. Liu, M. Zhang and J. Zhang, Chem. Commun., 2011, 47, 12870 RSC; (h) J. Zhang, Y. Xiao and J. Zhang, Adv. Synth. Catal., 2013, 355, 2793 CrossRef CAS PubMed.
- J.-K. Qiu, W.-J. Hao, D.-C. Wang, P. Wei, J. Sun, B. Jiang and S.-J. Tu, Chem. Commun., 2014, 50, 14782 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1410586 (3a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra13319j |
| ‡ These authors have equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2015 |