Tailoring the electrical properties of multilayer MoS2 transistors using ultraviolet light irradiation†
Abstract
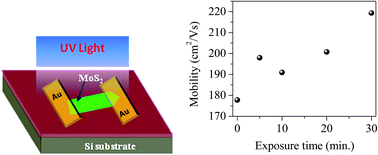
Two dimensional layered semiconductor transition-metal dichalcogenides such as molybdenum disulfide (MoS2) have attracted tremendous interest as a new class of electronic material and generated considerable attention as a promising channel material for field-effect transistors (FETs). Modulating the electronic properties of MoS2 is essential in order to obtain the best performance of its electronic and optoelectronic devices as well as enabling fabrication of various complex devices. In this paper we report a very straightforward and simple technique to tailor the performance of multilayer (ML) MoS2 FETs by exposure to nitrogen (N2) gas under deep-ultraviolet (DUV) light. Threshold voltages of ML MoS2 FETs shifted towards a negative gate voltage after exposure to N2 gas in the presence of DUV light. We also observed that drain-to-source current, carrier density and charge-carrier mobility of ML MoS2 are significantly improved after exposure to N2 gas under DUV light irradiation.

 Please wait while we load your content...
Please wait while we load your content...