Ferromagnetism of three-dimensional graphene framework†
Sai Qina,
Pingping Suna,
Qitao Dib,
Shuang Zhoua,
Caiping Yanga and
Qingyu Xu*acd
aDepartment of Physics & Key Laboratory of MEMS of the Ministry of Education, Southeast University, Nanjing 211189, China. E-mail: xuqingyu@seu.edu.cn
bDepartment of Materials Science and Engineering, Nanjing University, Nanjing 210008, China
cNational Laboratory of Solid State Microstructures, Nanjing University, Nanjing 210093, China
dCollaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, China
First published on 23rd October 2015
Abstract
A three-dimensional graphene framework (3DGF) was synthesized via hydrothermal growth of graphene oxide (GO) suspension. The structure of 3DGF was characterized by scanning electron microscope, Raman spectroscopy and X-ray photoelectron spectroscopy. The reduction of GO with increased defect density has been confirmed, promoting the formation of 3D pore structure. The magnetic properties of 3DGF were investigated experimentally. Pure diamagnetism was observed in GO, while weak ferromagnetism was observed in 3DGF with saturated ferromagnetic magnetization of 0.005 emu g−1 at 300 K. The observed ferromagnetism has been attributed to the presence of increased concentration of sp3-type hydroxyl groups attached on the basal plane at the interconnected 3D porous network structure of 3DGF.
1. Introduction
Graphene, a single layer of sp2-hybridized carbon atoms arranged in a two-dimensional hexagonal lattice, has attracted tremendous attention since its discovery in 2004.1 Graphene has potential applications in various fields such as electronic devices,2 composites,3 catalysis,4 bio-sensors,5 supercapacitors,6 Li ion batteries7 and hydrogen storage,8 mainly due to its superior electronic, thermal, mechanical properties, and excellent chemical stability. However, ideal graphene is nonmagnetic itself, and the localized magnetic moments are absent owing to a delocalized π bonding network, which limits its applications in spintronics devices.9Traditional magnetic materials have been associated with the elements containing partially filled 3d or 4f subshells which possess the localized magnetic moments,10 magnetism appearing in graphene-based materials has attracted extensive attention because graphene contains only sp electrons. Theoretical studies confirmed that defects such as vacancies,11 adatoms,12 and zigzag edges13 can introduce localized magnetic moments. Experimentally, many approaches have been used to produce magnetism in graphene, including dopant atoms (H14, N15 and F16) and edge defects.17 Up to now, the reported magnetic researches on graphene-based materials still concentrated primarily on two-dimensional (2D) graphene. 2D graphene sheets could be self-assembled into complex three dimensional (3D) macrostructures. 3D graphene not only possesses intrinsic properties of 2D graphene sheets, but also provides advanced functions with improved performance in various applications, such as supercapacitors18 and energy storage,19 etc. However, the magnetic properties of 3D graphene have not yet been studied. In this paper, we report the magnetic properties of 3D graphene framework (3DGF), which was prepared by a facile one-step hydrothermal method, without any additives excepted for graphene oxide (GO) suspension.20 Weak ferromagnetism with saturated magnetization of 0.005 emu g−1 at 300 K has been observed. The reduction of GO with high concentration of sp3-type hydroxyl groups attached to the basal plane in the interconnected 3D porous network structure has been confirmed, which is believed to be the origin of magnetism.
2. Experimental details
GO was synthesized by the oxidation of natural graphite powder (325 mesh) according to a modified Hummers' method.21 The obtained GO was repeatedly washed by diluted hydrochloric acid several times to totally remove the possible remaining 3d transition metal contaminations. 3DGF was prepared based on the following procedure.20 A 10 mL 2 mg mL−1 GO aqueous dispersion was sealed in a 23 mL Teflon-lined autoclave without any additives and maintained at 180 °C for 6 h. Then the autoclave was naturally cooled to room temperature. Finally, 3DGF was obtained by freeze-drying.The morphologies of the samples were observed by a scanning electron microscope (SEM, FEI Inspect F50). Raman measurements were performed on a Jobin Yvon LabRAM HR800 micro-Raman spectrometer using a laser excitation of 514 nm at room temperature. The electronic states were studied by X-ray photoelectron spectroscopy (XPS, ThermoFisher SCIENTIFIC) measurements using Al Kα X-ray source (hν = 1486.6 eV). The magnetic properties were measured by a vibrating sample magnetometer (VSM) integrated in a physical property measurement system (PPMS-9, Quantum Design).
3. Results and discussion
A homogeneous aqueous mixture of GO (2 mg mL−1) allows the assembly of 3D porous graphene networks (Fig. 1a). The as-prepared 3DGF is so strong that it can be obtained as a free-standing cylinder. This has been attributed to the partial overlapping or coalescing of flexible graphene sheets via π–π stacking interactions during the hydrothermal reduction, which forms the strong cross-links of 3D graphene network.20 SEM images reveal that the freeze-dried 3DGF has a well-defined and interconnected 3D porous network with pore sizes ranging from sub-micrometers to several micrometers and pore walls consisting of thin layers of stacked graphene sheets (Fig. 1b). During the reduction of GO, GO sheets changed from hydrophilic to hydrophobic. The combination of hydrophobic and π–π interactions caused a 3D random stacking between flexible graphene sheets.20,22 Raman spectroscopy is an essential tool to characterize carbonaceous materials with ordered and disordered carbon structures. The Raman spectra of GO and 3DGF are shown in Fig. 1c. Two distinct peaks of 1350 and 1595 cm−1 can be observed, which are assigned to the D and G band of graphene material, respectively. The G band corresponding to the first-order scattering of the E2g mode symmetry is a characteristic feature of the graphitic layers,23 while the D band due to the κ-point phonons of A1g indicates the formation of defects and disorder such as the presence of in-plane hetero-atoms, grain boundaries, aliphatic chain, etc.24 The relative intensity of D band compared to G band is often used to evaluate the defects density in graphene.25 The higher D/G ratio in Raman spectra observed in 3DGF than GO can be attributed to additional defects, this agrees well with the previous report.26 In addition, the intensity ratio of D and G bands (ID/IG) can also be utilized to evaluate the in-plane crystallite sizes (La) of graphene materials. The crystallite size can be determined based on the Tuinstra–Koenig relationship:| La (nm) = (2.4 × 10−10) × λ4 × (ID/IG)−1 | (1) |
 | ||
| Fig. 1 (a) Photographs of 3DGF (b) SEM images of 3DGF interior microstructures. (c) Raman spectra of GO and 3DGF. | ||
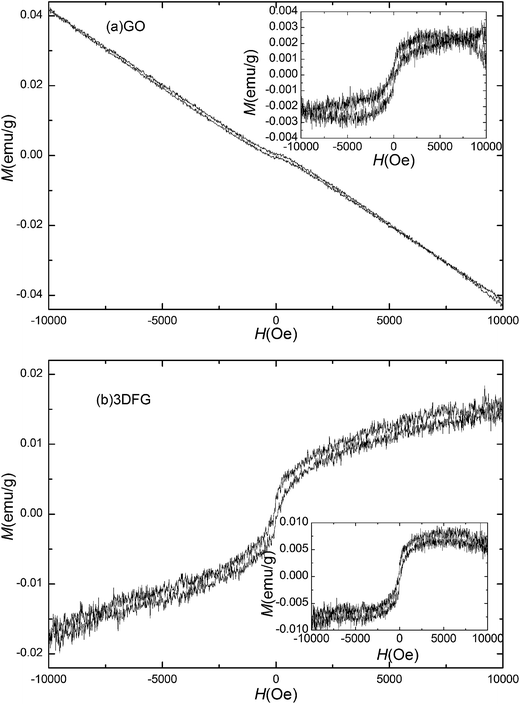
The field dependent magnetization (M–H) curves of GO and 3DGF were measured at room temperature in the field range of −10 kOe < H < 10 kOe, shown in Fig. 2a. The linear M–H curves with negative slope for the VSM rod and sample holder is shown in Fig. S1 (in the ESI†), demonstrating the pure diamagnetic background which has been subtracted in the magnetic data of the samples. Thus the possible ferromagnetic artifacts from the measuring system can be safely excluded. The linear M–H curve with negative slope suggests the diamagnetic properties of GO without any ferromagnetic signals. Besides the main diamagnetic background of 3DGF, a slightly S-shaped curve at low field can be observed in the M–H curve (Fig. 2b), suggesting the dominant diamagnetism with weak ferromagnetism. After subtracting the diamagnetic background, the ferromagnetic contribution can be obtained with saturated ferromagnetic magnetization (Ms) of 0.005 emu g−1, as shown in the inset, which is comparable to the value of reduced GO (photo-irradiated GO for 6 h) reported by Taniguchi et al. (0.004 emu g−1 at room temperature)28 and hydrogenated graphene reported by Eng et al. (0.006 emu g−1) previously.29 It should be noted that the sensitivity of our VSM is in the order of 10−6 emu. The raw data of M–H curves for GO and 3DGF is shown in Fig. S2 (in the ESI†), and the ferromagnetic signal is one order larger than the sensitivity. To study the difference between the magnetic properties of 3DGF and 2D graphene, the rGO900 (2D graphene) was synthesized from GO by annealing at 900 °C in Ar atmosphere for 1 h. The M–H curve for rGO900 at 300 K is shown in Fig. S3 (in the ESI†). Ferromagnetism with a saturation magnetization of 0.0035 emu g−1 at room temperature was found in the rGO900, a little lower than 3DGF (0.005 emu g−1). We suggest that the stronger ferromagnetism in 3DGF might be due to 3D porous network structure, which needs further study. The observed ferromagnetism in graphene was generally very weak, which has been attributed to the large distance between the adjacent magnetic moments in graphene, leading to the too weak inter-cluster exchange interaction and hindering the formation of long-range magnetic ordering.30 Fig. 3 shows M–H curves for GO and 3DGF at 5 K, with ferromagnetic magnetization shown in the insets. Interestingly, GO also exhibits weak ferromagnetism, which is generated by the interaction between separated magnetic regions and domains, this agrees with the previous report.28 The negative slope at high field indicates the main diamagnetism in GO even at 5 K. Interestingly, the slope at high fields changes to positive for 3DGF. This suggests the high concentration of mainly isolated magnetic functional groups in 3DGF which suppress the diamagnetic contribution from GO matrix. The saturated ferromagnetic magnetization Ms of GO and 3DGF reaches 0.003 and 0.0085 emu g−1, respectively. Compared with GO, the saturated ferromagnetic magnetization of 3DGF is significantly enhanced. The temperature-dependent magnetization (M–T) measurements for GO and 3DGF are shown in Fig. 4, which were measured in the presence of 1 kOe during the warming processes to 300 K after cooling down from 300 K under 1 kOe. For GO, the magnetization is negative above 13 K, which shows the diamagnetic properties of GO. The M–T curve of 3DGF shows two-step behavior, M starts to increase with decreasing T at around 100 K, and a drastic increase of M with decreasing T can be observed below 20 K. The transition at around 100 K is due to that a disordered magnetism exists where different magnetic correlations compete with each other.31–33 The transition at 20 K indicates that paramagnetic behavior exists in 3DGF. The nearly constant magnetization up to 300 K further confirms the weak ferromagnetism in 3DGF and suggests that the Curie temperature of 3DGF is much higher than room temperature. GO contains many hydroxyl, carboxyl, epoxy, carbonyl groups on the main graphene skeleton.34 Via one-step hydrothermal process, some of these groups were removed, and some of the damaged sp2 carbon conjugations were restored. The restored conjugated structure generated partial overlapping or coalescing of flexible graphene sheets with greatly increased amount of π–π stacking sites, which resulted in the formation of physical cross-linking sites of the 3D framework of 3DGF.20,35 The interconnected 3D porous network structure indicates the possible existence of high density of defects in the 3DGF.
 | ||
| Fig. 2 M–H curves for (a) GO and (b) 3DGF at 300 K. Insets show the ferromagnetic magnetization of 3DGF after subtracting the diamagnetic background. | ||
 | ||
| Fig. 3 M–H curves for (a) GO and (b) 3DGF at 5 K. Insets show the ferromagnetic magnetization after subtracting the high field linear background. | ||
To examine the composition of GO and 3DGF, XPS measurements were carried out. In the XPS spectra of GO (Fig. 5a) and 3DGF (Fig. 5b), the peaks appeared at about 284.6 and 532.3 eV corresponding to C 1s and O 1s. XPS studies show that the C/O atomic ratio is 2.3 for GO and 6.4 for 3DGF, respectively. Fig. 5c are the high-resolution C 1s peak of GO, which was deconvoluted into three types of carbon bonds at 284.6, 286.7, 287.7 eV, which correspond to C–C, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. The C 1s XPS spectrum of 3DGF (Fig. 5d) also exhibits the same peaks. Compared with the spectrum of GO, the peak intensities of the oxygen functional groups of 3DGF are much weaker. The decreasing concentration of oxygen functional groups reflects the reduction of GO to graphene by hydrothermal method. The ‘non-magnetic’ unstable oxygen groups in GO are removed and some ‘magnetic’ stable groups are left, resulting the existence of ferromagnetism in 3DGF.
O, respectively. The C 1s XPS spectrum of 3DGF (Fig. 5d) also exhibits the same peaks. Compared with the spectrum of GO, the peak intensities of the oxygen functional groups of 3DGF are much weaker. The decreasing concentration of oxygen functional groups reflects the reduction of GO to graphene by hydrothermal method. The ‘non-magnetic’ unstable oxygen groups in GO are removed and some ‘magnetic’ stable groups are left, resulting the existence of ferromagnetism in 3DGF.
To further understand which oxygen group plays the important role in the magnetic properties of graphene, the typical O 1s spectra of GO and 3DGF are shown in Fig. 6. Both spectra were decomposed into three main components; OH (hydroxyl C–O) near 531 eV, epoxy/ether (aliphatic C–O) around 523.3 eV, and carbonyl (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 533.4 eV.30,36 The peak intensity ratio of epoxy group in oxygen bondings in 3DGF is significantly less than that of GO, suggesting that epoxy group is not the origin of ferromagnetism in 3DGF. It is known that the carbonyl groups usually located at sheet edges, the contribution to magnetic moments by carbonyl groups is insignificant.37 As for OH groups, they can covalently bond to the basal-plane carbon atoms to form sp3-type defects, not only limited to edge or vacancy sites.30 In addition, XPS peak intensity of OH group in oxygen bondings in 3DGF is obviously larger than the other oxygen groups. The ratios of OH, epoxy/ether and carbonyl groups to carbon for GO and 3DGF are shown in Table 1. It can be clearly seen that the concentration of OH groups is significantly increased while the concentrations of the other two groups are obviously reduced. The results definitely suggest that the bonded OH groups in 3DGF contribute to the magnetic moments.
O) at 533.4 eV.30,36 The peak intensity ratio of epoxy group in oxygen bondings in 3DGF is significantly less than that of GO, suggesting that epoxy group is not the origin of ferromagnetism in 3DGF. It is known that the carbonyl groups usually located at sheet edges, the contribution to magnetic moments by carbonyl groups is insignificant.37 As for OH groups, they can covalently bond to the basal-plane carbon atoms to form sp3-type defects, not only limited to edge or vacancy sites.30 In addition, XPS peak intensity of OH group in oxygen bondings in 3DGF is obviously larger than the other oxygen groups. The ratios of OH, epoxy/ether and carbonyl groups to carbon for GO and 3DGF are shown in Table 1. It can be clearly seen that the concentration of OH groups is significantly increased while the concentrations of the other two groups are obviously reduced. The results definitely suggest that the bonded OH groups in 3DGF contribute to the magnetic moments.
| OH/C | (Epoxy/ether)/C | Carbonyl/C | |
|---|---|---|---|
| GO | 2.15 at% | 36.8 at% | 4.53 at% |
| 3DGF | 7.42 at% | 5.15 at% | 3.05 at% |
When ferromagnetism is observed in graphene-based materials, it is often questioned whether the ferromagnetic signals come from magnetic impurities. To check the possible contribution from magnetic impurities, XPS and inductively coupled plasma (ICP) spectrometry were performed. XPS is not a sensitive method for the trace element. The 3d impurity elements of all the samples are lower than the detection limit from the Fe 2p, Co 2p, Ni 2p XPS spectra (not shown here). The highly sensitive technique of ICP analysis was performed, the total amount of magnetic impurities (Fe, Co, Ni) in all the samples was determined to be below 2 ppm. An Fe impurity content of 1 μg g−1 would give the magnetization of 2.2 × 10−4 emu g−1,38 the observed ferromagnetic magnetization for 3DGF (0.005 emu g−1 at 300 K) should correspond to 23 ppm of Fe (or 167 ppm Ni and 63 ppm Co).29 These results demonstrate the observed ferromagnetism in 3DGF originates from the possible existence of high density of sp3-type hydroxyl defects (OH group) at the interconnected 3D porous network structure instead of magnetic impurities.
4. Conclusions
In summary, we have observed the room temperature ferromagnetism of 3DGF obtained from 2D soluble graphene oxide sheets through one-step hydrothermal method. During the hydrothermal process, some of oxygen functional groups were removed, the restored conjugated structure of GO sheets forms the strong cross-links of the 3D graphene network, high concentration of sp3-type hydroxyl defects (OH group) are introduced in GO, which might be the source of the room temperature ferromagnetism observed in 3D porous graphene sheets network structure. Our results might stimulate more extensive studies on magnetism of three dimensional graphene-based materials and their related spintronics applications.Acknowledgements
This work is supported by the National Natural Science Foundation of China under Grant number 51172044 and 51471085, the Natural Science Foundation of Jiangsu Province of China (BK20151400).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed.
- S. Stankovich, D. A. Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura and I. Honma, Nano Lett., 2009, 9, 2255–2259 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- H. F. Xiang, Z. D. Li, K. Xie, J. Z. Jiang, J. J. Chen, P. C. Lian, J. S. Wu, Y. Yu and H. H. Wang, RSC Adv., 2012, 2, 6792 RSC.
- J. Zhang, X. Liu, H. Zhou, X. Yan, Y. Liu and A. Yuan, RSC Adv., 2014, 4, 28908 RSC.
- L. Li, R. Qin, H. Li, L. Yu, Q. Liu, G. Luo, Z. Gao and J. Lu, ACS Nano, 2011, 5, 2601–2610 CrossRef CAS PubMed.
- D. C. Yan, S. Y. Chen, M. K. Wu, C. C. Chi, J. H. Chao and M. L. H. Green, Appl. Phys. Lett., 2010, 96, 242503 CrossRef.
- O. V. Yazyev and L. Helm, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125408 CrossRef.
- J. Zhou, Q. Wang, Q. Sun, X. S. Chen, Y. Kawazoe and P. Jena, Nano Lett., 2009, 9, 3867–3870 CrossRef CAS PubMed.
- S. Bhowmick and V. B. Shenoy, J. Chem. Phys., 2008, 128, 244717 CrossRef PubMed.
- L. Xie, X. Wang, J. Lu, Z. Ni, Z. Luo, H. Mao, R. Wang, Y. Wang, H. Huang, D. Qi, R. Liu, T. Yu, Z. Shen, T. Wu, H. Peng, B. Özyilmaz, K. Loh, A. T. S. Wee, Ariando and W. Chen, Appl. Phys. Lett., 2011, 98, 193113 CrossRef.
- Y. Liu, N. Tang, X. Wan, Q. Feng, M. Li, Q. Xu, F. Liu and Y. Du, Sci. Rep., 2013, 3, 2566 Search PubMed.
- Q. Feng, N. Tang, F. Liu, Q. Cao, W. Zheng, W. Ren, X. Wan and Y. Du, ACS Nano, 2013, 7, 6729–6734 CrossRef CAS PubMed.
- Z. J. Yue, D. H. Seo, K. Ostrikov and X. L. Wang, Appl. Phys. Lett., 2014, 104, 092417 CrossRef.
- Z. S. Wu, A. Winter, L. Chen, Y. Sun, A. Turchanin, X. Feng and K. Mullen, Adv. Mater., 2012, 24, 5130–5135 CrossRef CAS PubMed.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. Yang, X. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- L. Zhang and G. Shi, J. Phys. Chem. C, 2011, 115, 17206–17212 CAS.
- H. Kim, J. Cho, S. Y. Jang and Y. W. Song, Appl. Phys. Lett., 2011, 98, 021104 CrossRef.
- K. Krishnamoorthy, M. Veerapandian, K. Yun and S. J. Kim, Carbon, 2013, 53, 38–49 CrossRef CAS.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS PubMed.
- Y. Xu, Z. Lin, X. Zhong, X. Huang, N. O. Weiss, Y. Huang and X. Duan, Nat. Commun., 2014, 5, 4554 CAS.
- L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago and M. A. Pimenta, Appl. Phys. Lett., 2006, 88, 163106 CrossRef.
- T. Taniguchi, H. Yokoi, M. Nagamine, H. Tateishi, A. Funatsu, K. Hatakeyama, C. Ogata, M. Ichida, H. Ando, M. Koinuma and Y. Matsumoto, J. Phys. Chem. C, 2014, 118, 28258–28265 CAS.
- A. Y. S. Eng, H. L. Poh, F. Sanek, M. Marysko, S. Matejkova, Z. Sofer and M. Pumera, ACS Nano, 2013, 7, 5930–5939 CrossRef CAS PubMed.
- T. Tang, N. Tang, Y. Zheng, X. Wan, Y. Liu, F. Liu, Q. Xu and Y. Du, Sci. Rep., 2015, 5, 8448 CrossRef CAS PubMed.
- P. Sun, K. Wang, J. Wei, M. Zhong, D. Wu and H. Zhu, Nano Res., 2014, 7, 1507–1518 CrossRef CAS.
- H. S. S. R. Matte, K. S. Subrahmanyam and C. N. R. Rao, J. Phys. Chem. C, 2009, 113, 9982–9985 CAS.
- Y. Kopelevich, R. R. da Silva, J. H. S. Torres, A. Penicaud and T. Kyotani, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 092408 CrossRef.
- S. Park and R. S. Ruoff, Nat. Nanotechnol., 2009, 4, 217–224 CrossRef CAS PubMed.
- K. Sheng, Y. Xu, C. Li and G. Shi, New Carbon Mater., 2011, 26, 9–15 CrossRef CAS.
- A. Ganguly, S. Sharma, P. Papakonstantinou and J. Hamilton, J. Phys. Chem. C, 2011, 115, 17009–17019 CAS.
- T. Tang, F. Liu, Y. Liu, X. Li, Q. Xu, Q. Feng, N. Tang and Y. Du, Appl. Phys. Lett., 2014, 104, 123104 CrossRef.
- P. Esquinazi, A. Setzer, R. Höhne, C. Semmelhack, Y. Kopelevich, D. Spemann, T. Butz, B. Kohlstrunk and M. Lösche, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 024429 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra14377b |
| This journal is © The Royal Society of Chemistry 2015 |



