Hybrids based on transition metal phosphide (Mn2P, Co2P, Ni2P) nanoparticles and heteroatom-doped carbon nanotubes for efficient oxygen reduction reaction†
Kuiyong Chenab,
Xiaobin Huang*a,
Chaoying Wanc and
Hong Liua
aSchool of Aeronautics and Astronautics, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, PR China. E-mail: xbhuang@sjtu.edu.cn; Fax: +86-21-54741297; Tel: +86-21-54747142
bSchool of Materials Science and Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, PR China
cInternational Institute for Nanocomposites Manufacturing, WMG, University of Warwick, Coventry, CV4 7AL, UK
First published on 22nd October 2015
Abstract
Hybrids based on transition metal phosphide (Mn2P, Co2P, Ni2P) nanoparticles and heteroatom-doped carbon nanotubes were facilely synthesized, and used as efficient oxygen reduction reaction (ORR) catalysts in alkaline solution. Transition metal phosphide nanoparticles formed core/shell structures with graphitic carbon, and the nanoparticles (core) can significantly influence the ORR catalytic activity of the carbon shell. Hybrids based on Co2P and Mn2P decorated heteroatom-doped carbon exhibit excellent ORR catalytic activity with regards to dominant 4e− process, low over potential, excellent methanol tolerance and durability. In contrast, Ni2P decorated heteroatom-doped carbon shows inferior ORR catalytic activity. The variation of ORR performance mainly derives from the collective effect of the electronegativity and binding energy shift of the transition metals, which would result in a different surface electronic structure of the heteroatom-doped carbon. Low electronegativity and low binding energy shift of the transition metals would lead to strong electron-donating ability of the transition metal phosphide nanoparticles, resulting in the enhanced ORR catalytic activity of the hybrid materials. This work is significant for development of advanced ORR catalysts based on heteroatom-doped carbon via rational design of the structure of hybrid materials.
1. Introduction
Heteroatom (B, N, O, S, P or their combination)-doped carbon materials are extensively studied as efficient catalysts in the ORR.1–8 Because of their long term stability, good conductivity, low cost, and relatively high activity, heteroatom-doped carbon materials have been demonstrated as potential substitutes for noble metal based catalysts (e.g. Pt/C). However, heteroatom-doped carbons are still on a less competitive level compared to commercialized Pt/C catalysts in the aspects of typical 4e− process and positive onset potential etc. The development of new carbon-based catalysts with enhanced oxygen reduction activity is of great importance.ORR catalytic activity of heteroatom-doped carbon materials is significantly affected by the surface electronic structure of carbon materials. Doping of carbon materials with heteroatoms can disturb the electroneutrality of the surface,9,10 and render active sites on the surface of carbon materials.11,12 Furthermore, it is of great interest and significance for further tailoring the electronic inhomogeneity of the doped carbon materials to develop ORR catalysts with enhanced activity.13–17 In our previous study, heteroatom-doped carbon nanotubes decorated with cobalt phosphide (Co2P) nanoparticle were facilely prepared and used as ORR catalyst.18 The electronic interaction between the Co2P nanoparticles and the heteroatom-doped carbon structures synergistically prompt the ORR catalytic performance of the carbon nanotubes. However, the functions and influences of the transition metals on the ORR performance of the hybrid catalysts have not yet been revealed. It is a critical step to figure out the influence of transition metals for rational design high performance ORR catalysts. Motivated by this, the present work provides the preparation of heteroatom-doped carbon nanotubes decorated with different transition phosphide (Mn2P, Co2P, Ni2P) nanoparticles, and study the effect of the metals on the surface electronic structure and the ORR catalytic activity of the heteroatom-doped carbon.
Herein, heteroatom-doped carbon nanotubes decorated with M2P (M = Co, Mn, Ni) nanoparticles were synthesized via carbonization of the composite of transition metal precursors and polyphosphazene coated multi-walled carbon nanotubes (MWCNTs). For the good electrical conductivity and stable physical and chemical properties, MWCNTs show of great potential for electro energy storage and conversion, however, their inert surface limits their large-scale applications. Coating of MWCNTs with polyphosphazene can change the inert surface of carbon nanotubes, and introduce heteroatoms to the final carbon materials. Meanwhile, polyphosphazene provides phosphorus to form M2P (M = Co, Mn, Ni) nanoparticles. The content of heteroatoms in the as prepared hybrid materials is as high as 10 at%, and the surface areas of the hybrid materials are much higher than that of pristine MWCNTs. Hybrids based on Co2P and Mn2P decorated carbon nanotubes show significantly enhanced ORR catalytic activity than heteroatom-doped carbon, as well as superior methanol tolerance and durability than commercial Pt/C catalysts, indicating their great potential for use as efficient catalysts in energy conversation and storage fields.
2. Experimental
2.1 Synthesis of polyphosphazene coated MWCNTs (CNTs@PPA)
CNTs@PPA was synthesized via a solvothermal method. Typically, 400 mg MWCNTs were dispersed in 20 ml acetonitrile (AN) by ultrasonic treatment for 1 hour. The solution was transferred to an autoclave containing 400 mg hexachlorocyclotriphosphazene (HCCP) and 400 mg p-phenylenediamine (p-PDA). After addition of 2 ml of TEA and saturated with nitrogen, the autoclave was sealed and maintained at 150 °C for 3 hours. The final product was washed with ethanol and water, respectively, and freeze-dried.2.2 Synthesis of CNTs@PPA loaded with transition metal precursors (CNTs@PPA–M (M = Co, Mn, Ni))
For the synthesis of CNTs@PPA–Co, 0.1 g CNTs@PPA was dispersed in 10 ml n-propanol. 0.1 ml of 0.6 M cobaltous acetate aqueous solution was added to the CNTs@PPA/n-propanol suspension. After dispersion by ultrasound irradiation for 1 hour, 0.1 ml of NH4OH (30% solution) was added to the solution, and the solution was maintained for 30 min. Ultimately, CNTs@PPA–Co was obtained by centrifugation, washing with deionized water and freeze-dried.The synthesis of CNTs@PPA–Mn was similar to that of CNTs@PPA–Co, besides manganese acetate was used as transition metal precursor.
For the synthesis of CNTs@PPA–Ni, 0.1 g CNTs@PPA was dispersed in 10 ml n-propanol. 0.3 ml of 0.2 M nickelous acetate aqueous solution was added to the CNTs@PPA/n-propanol suspension. After dispersion by ultrasound irradiation, the solution was maintained at 80 °C for 3 hours. Ultimately, CNTs@PPA–Ni was obtained by centrifugation, washing with deionized water and freeze-dried.
2.3 Synthesis of transition metal phosphide modified heteroatom-doped carbon nanotubes (HCNT–M2P (M = Co, Mn, Ni))
HCNT–M2P (M = Co, Mn, Ni) was prepared by direct carbonization of CNTs@PPA–M (M = Co, Mn, Ni) in a tube type resistance furnace under an inert atmosphere. In a typical procedure, 0.20 g of CNTs@PPA–M in a ceramic boat was putted in the furnace. The temperature was ramped to 500 °C with a rate of 5 °C min−1 under a flow of pure N2. Followed by an isothermal process for 5 hours, the temperature was ramped to 900 °C at a heating rate of 5 °C min−1, and kept at that value for 2 hours. Samples were collected when the resistance furnace was cooled to ambient temperature. Final products are denoted as HCNT–M2P (M = Co, Mn, Ni).For comparison, CNTs@PPA was carbonized under similar condition to prepare heteroatom-doped carbon nanotubes without any transition metal compounds, and the final product was denoted as HCNTs.
2.4 Electrochemical measurements
Electrochemical measurements were conducted using a conventional three-electrode cell filled with 0.1 M KOH solution. Platinum wire and Ag/AgCl electrodes were used as the reference and counter electrodes, respectively. The catalyst ink was prepared by ultrasonically dispersing 5 mg catalyst powder in 1 ml of ethanol and high-purity water (volume ratio, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) mixture in the presence of 50 μl Nafion solution (5 wt%) for 30 min. Then 10 μl of this ink was deposited onto the disk electrode and air-dried for use. CVs and RDE experiments were performed in oxygen-saturated 0.1 M KOH solution at the potential scanning rate of 10 mV s−1. The potential ranges for CV and RDE range from 0 V to −1.0 V and 0.1 to −1.0 V (vs. Ag/AgCl), respectively.
2) mixture in the presence of 50 μl Nafion solution (5 wt%) for 30 min. Then 10 μl of this ink was deposited onto the disk electrode and air-dried for use. CVs and RDE experiments were performed in oxygen-saturated 0.1 M KOH solution at the potential scanning rate of 10 mV s−1. The potential ranges for CV and RDE range from 0 V to −1.0 V and 0.1 to −1.0 V (vs. Ag/AgCl), respectively.
3. Results and discussion
As shown in Fig. 1, the preparation of HCNT–M2P (M = Co, Mn, Ni) mainly involves the one-pot solvothermal synthesis of CNTs@PPA, followed by the loading of transition metal precursors, and the carbonization process. The morphology of CNTs@PPA was characterized by transmission electron microscopy (TEM). MWCNTs were uniformly coated with polyphosphazene, and the thickness could be altered by change the mass ratio between MWCNTs and the comonomers (Fig. S1, ESI†). After heat treatment of CNTs@PPA–M (M = Co, Mn, Ni) at 900 °C under nitrogen atmosphere, polyphosphazene was transformed into amorphous carbon, and uniform transition metal phosphide nanoparticles were formed on the surface of heteroatom-doped carbon nanotubes (Fig. 2). In contrast, the transition metal compound supported on the pristine MWCNTs shows poor dispersion (Fig. S2, ESI†). It is probably due to the coordination behavior between polyphosphazene and transition metal compounds, leading to uniformly dispersed transition metal phosphide nanoparticles.19 Furthermore, as shown in Fig. 2(b, d and f), the transition metal phosphide nanoparticles were covered with graphitic carbon, and formed core/shell structures. High resolution TEM (HRTEM) images of the Co2P, Mn2P and Ni2P nanoparticles can be found in Fig. 2(b, d and f). The lattice fringes suggest good crystallinity of the M2P nanoparticles (M = Co, Mn, Ni), which are in accordance with the corresponding XRD patterns (Fig. 3). | ||
| Fig. 2 TEM images of HCNT–Co2P (a and b), HCNT–Mn2P (c and d) and HCNT–Ni2P (e and f) at various magnifications. The insets in (b, d and f) show the HRTEM images of Co2P, Mn2P and Ni2P, respectively. | ||
The crystalline properties of HCNT–M2P were analyzed using X-ray diffraction (XRD) method. In Fig. 3(a), the emergency of characteristic peaks at 2θ = 40.7, 43.3° indicates the formation of Co2P nanoparticles with hexagonal phase (PDF 32-0306). In Fig. 3(b), the diffraction peaks at 2θ = 39.4, 43.2, 45.5° indicate the formation of Mn2P nanoparticles (PDF 65-3545). The diffraction peaks at 2θ = 40.8, 44.6, 47.3, 54.2, 74.7° in Fig. 3(c) indicate the formation of Ni2P nanoparticles (PDF 03-0953). In addition, the diffraction peaks at 2θ = 26.6, 42.4° in Fig. 3(a–c) can be ascribed to the (002) and (101) reflection planes of MWCNTs, indicating the graphitic structure of the carbon nanotubes. Furthermore, in comparing with the XRD pattern of MWCNTs (Fig. 3(d)), the broad diffraction uplifts at 2θ = 22–25° in Fig. 3(a–c) suggest some turbostratic carbon was introduced. It is due to that the polyphosphazene was transformed into turbostratic carbon at high temperature.18,20 The increased intensity ratios of D and G lines in Raman spectra also suggest some turbostratic carbon was introduced (Fig. S3, ESI†).21–23
Nitrogen adsorption–desorption measurements were conducted to characterize the surface structure of HCNT–M2P (M = Co, Mn, Ni). Nitrogen adsorption–desorption isotherms and pore size distribution curves of the samples are shown in Fig. S4 (ESI†). The specific surface areas of the samples were calculated through the BET (Brunauer–Emmett–Teller) method. Data on the textural properties of the samples are shown in Table 1. In compare with MWCNTs (with BET surface area ∼70 m2 g−1), HCNT–M2P (M = Co, Mn, Ni) show higher BET surface area, due to the introduction of amorphous carbon in the carbonization process. XPS was used to study the surface chemical compositions of HCNT–M2P (M = Co, Mn, Ni). Wide-region-scanning XPS spectra are shown in Fig. S5 (ESI†). Surface of all samples were found to be composed of C, N, O, P and the corresponding transition metal, and the level of heteroatoms could reach as high as 10 at% (Table 1). Because of high level of heteroatoms, Raman response of HCNT–M2P (M = Co, Mn, Ni) shows a red-shift for both D and G line, reflecting the n-type doping (N, P, O) structure of the carbon materials (Fig. S3, ESI†).24,25 High content of heteroatom-doped structure could effectively change the surface structure of the carbon substrates.26
| Sample | C (at%) | N (at%) | O (at%) | P (at%) | Co (at%) | Mn (at%) | Ni (at%) | SBETa m2 g−1 | Vporeb cm3 g−1 |
|---|---|---|---|---|---|---|---|---|---|
| a Specific surface area calculated by BET method.b Pore volume of the samples. | |||||||||
| HCNT–Co2P | 90.23 | 1.63 | 5.98 | 2.04 | 0.12 | 0 | 0 | 267.3 | 0.338 |
| HCNT–Mn2P | 89.46 | 1.62 | 6.80 | 1.96 | 0 | 0.16 | 0 | 277.6 | 0.255 |
| HCNT–Ni2P | 89.44 | 2.07 | 6.12 | 2.26 | 0 | 0 | 0.11 | 307.0 | 0.297 |
High-resolution XPS was used to study the chemical state and composition of M2P nanoparticles (M = Co, Mn, Ni). Fig. 4 shows the high-resolution XPS spectra of M2P (M = Co, Mn, Ni) in the M 2p and P 2p regions. Focusing initially on the XPS spectrum in Co 2p and P 2p regions of HCNT–Co2P (Fig. 4(a and b)), the peaks at 778.4 and 129.8 eV are assigned to the reduced Co and P species of Co2P nanoparticles, which are consistent with binding energies reported by others for Co (778.2 eV) and P (129.4–129.8 eV) in Co2P.27 The Co 2p3/2 binding energy of 778.8 eV is higher than that of Co metal (778.1–778.2 eV), while the P (2p) binding energy of 129.9 eV is lower than that of elemental phosphorus (130.2 eV), suggesting some degree of charge transfer from Co to P in Co2P.28 For HCNT–Mn2P and HCNT–Ni2P, the results of high resolution XPS also indicate some charge transfer from transition metal to phosphorus. As is shown in Fig. 4(d and f), the binding energy of P (2p) for both Mn2P and Ni2P are assigned to 129.9 eV, same to the P 2p for Co2P. Furthermore, the binding energy for Mn 2p3/2 (641.8 eV) in HCNT–Mn2P is shifted to a higher binding energy by 3.1 eV relative to that of Mn metal (Fig. 4(c)). The binding energy for Ni 2p3/2 (854.2 eV) in Ni2P is 1.5 eV higher than that of Ni metal (Fig. 4(e)). The XPS results suggest a charge transfer degree (δ) of the transition metals: δMn > δNi > δCo.
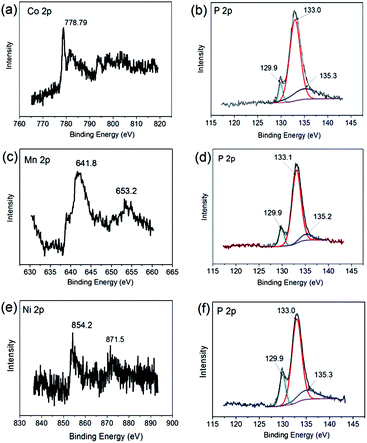 | ||
| Fig. 4 High-resolution XPS spectra of: HCNT–Co2P in the Co 2p (a) and P 2p regions (b), HCNT–Mn2P in the Mn 2p (c) and P 2p regions (d) and HCNT–Ni2P in the Ni 2p (e) and P 2p regions (f). | ||
The ORR catalytic performances of HCNT–M2P (M = Co, Mn, Ni) were characterized via cyclic voltammetry and linear sweep voltammetry (LSV) tests by using a rotating disk electrode (RDE). Fig. 5 demonstrates cyclic voltammogram (CV) plots of HCNT–M2P (M = Co, Mn, Ni) and HCNTs in N2- and O2-saturated 0.1 M KOH. The samples show featureless CV curves in oxygen free solution. In contrast, well defined redox peaks appear in O2-saturated solution, and the cathodic peaks can be logically attributed to the ORR process. Comparing with HCNTs, HCNT–M2P (M = Co, Mn, Ni) show more positive redox peaks, indicating the improvement of M2P (M = Co, Mn, Ni) to the ORR process of the heteroatom-doped carbon. In addition, Mn2P and Co2P nanoparticles decorated heteroatom-doped carbon nanotubes have more significant enhancement toward ORR catalytic ability than that of Ni2P nanoparticles decorated heteroatom-doped carbon nanotubes.
 | ||
| Fig. 5 Cyclic voltammograms of HCNTs (a), HCNTs–Mn2P (b), HCNTs–Co2P (c) and HCNTs–Ni2P (d) in O2-saturated and N2-saturated 0.1 M KOH solution. | ||
To gain further insight into the kinetics of the oxygen reduction reaction on HCNT–M2P (M = Co, Mn, Ni), LSV tests on RDE were performed in O2 saturated 0.1 M KOH solution at a scanning rate of 10 mV s−1. Fig. 6(a) shows the LSVs curves of pristine MWCNTs, HCNTs and HCNT–M2P (M = Co, Mn, Ni) on RDE in O2-saturated 0.1 M KOH at a rotation speed of 1800 rpm. The pristine MWCNTs showed low onset potential and typical two-plateaux process, indicating poor ORR catalytic activity of MWCNTs. It is probably due to lack of active cites on the surface of MWCNTs.29,30 HCNTs showed enhanced ORR activity than that of MWCNTs, due to the introduction of heteroatom-doped structure in the carbonization process. HCNT–Co2P and HCNT–Mn2P showed well-defined one-plateaux diffusion-limiting process below −0.3 V, indicating efficient surface electrocatalytic reaction. HCNT–Co2P and HCNT–Mn2P showed onset potentials about −0.12 and −0.11 V respectively, which are more positive than that of HCNTs (−0.15 V) and HCNT–Ni2P (−0.15 V). Furthermore, HCNT–Co2P and HCNT–Mn2P afforded half wave potentials of −0.19 and −0.18 V, respective, more positive than for HCNTs (−0.24 V) and HCNT–Ni2P (−0.24 V). The superior catalytic activity of HCNT–Co2P and HCNT–Mn2P than HCNTs suggested significant effect of Co2P and Mn2P nanoparticles. However, HCNT–Ni2P shows inferior catalytic activity than that of HCNT–Co2P and HCNT–Mn2P, and the activity is similar with that of HCNTs, indicating the inferior activity enhancement of Ni2P nanoparticles. Metal mass-specific LSV curves of HCNTs–Co2P, HCNTs–Mn2P, HCNTs–Ni2P are presented in Fig. S6 (ESI†). Mass of metals was estimated based on the XPS results. Similar to the results shown in Fig. 6(a), mass-specific LSV curves of HCNTs–Co2P and HCNTs–Mn2P showed more positive onset potentials than that of HCNTs–Ni2P. Meanwhile, HCNTs–Co2P displayed more positive half wave potential than that of HCNTs–Mn2P, HCNTs–Ni2P, suggesting outstanding catalytic activity of HCNTs–Co2P.
A set of polarization curves for the ORR on HCNT–M2P (M = Co, Mn, Ni) and HCNTs composite catalyst recorded from 500 to 1800 rpm are displayed in Fig. S7 and S8 (ESI†). The corresponding Koutecky–Levich plots (j−1 vs. ω−1/2) obtained from polarization curves at different potentials are shown as well. The plots at various electrode potentials exhibit good linearity and the slopes are approximately constant over the potential range between −0.3 and −0.8 V. The electron transfer number (n) per oxygen molecule was determined by the Koutecky–Levich equation (ESI†), and the results are shown in Fig. 6(b). The values of n for HCNT–Co2P and HCNT–Mn2P were calculated to be 3.52–3.78 over the potential range from −0.3 to −0.8 V, indicating a predominant 4e− ORR process, and most O2 molecules are directly reduced to OH−. The values of n for HCNTs–Ni2P are calculated to be 2.89–3.37, similar to that of HCNTs (2.83–3.33), indicating that the decoration with Ni2P has no obvious effect to the electron transfer number of the heteroatom-doped carbon materials.
As we have reported before, the synergistic effect via a unique host–guest electronic interaction between the transition metal phosphide nanoparticles and the graphitic carbon shell is of key importance to enhance the ORR catalytic activity of heteroatom-doped carbon. The embedded transition metal phosphide nanoparticles with stronger electron-donating ability would push the π electrons of heteroatom-doped graphitic carbon shell higher highest occupied molecular orbital (HOMO) levels and make the heteroatom-doped carbon shell more sensitive to oxidization, resulting in more enhanced catalytic activity of the heteroatom-doped carbon materials.
Herein, Co2P, Mn2P and Ni2P nanoparticles formed core/shell structure with graphitic carbon, and the nanoparticles set significant effect to the ORR catalytic activity of the heteroatom-doped carbon nanotubes. Specially, the strong electron-donating ability of Co2P makes HCNTs–Co2P a kind of excellent ORR catalyst.18 In comparing with Co (with electronegativity χCo = 1.88), Mn has lower electronegativity (χMn = 1.55), making Mn2P of great potential to have stronger electron-donating ability. However, Mn shows a larger charge transfer degree in the formation of Mn–P bond (XPS results, the binding energy shift of Mn in the formation Mn2P is +3.1 eV, higher than that of Co (+0.7 eV)), weakened the electron-donating ability of Mn2P nanoparticles. That makes the ORR catalytic activity of HCNTs–Mn2P similar to that of HCNTs–Co2P. Ni has the similar electronegativity (χNi = 1.91) compared with that of Co, however, the charge transfer degree of Ni in the formation of Ni–P bond is higher than that of Co (XPS results, the binding energy shift of Ni in the formation Ni2P is +1.5 eV). That leads to inferior catalytic performance of HCNTs–Ni2P than that of HCNTs–Co2P. Although the degree of charge transfer of Mn is higher than that of Ni in the formation of the corresponding transition metal phosphides, the lower electronegativity of Mn makes Mn2P of stronger electron-donating ability, which is the reason why HCNTs–Mn2P has superior activity than that of HCNTs–Ni2P. Both the electronegativity and binding energy have important effect on the electron-donating ability of the transition metal phosphides, and the ORR catalytic performance of heteroatom-doped carbon decorated with the corresponding transition metal phosphides.
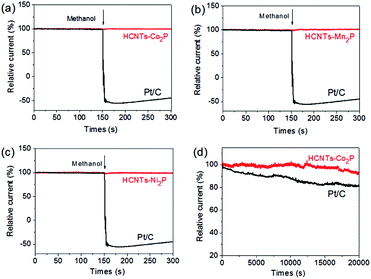
Chronoamperometric measurements were conducted to investigate possible methanol crossover effect and the electrochemical stability. As can be seen in Fig. 7(a–c), a distinct methanol oxidation current density for the Pt/C electrode was detected when 3 M methanol solution was added to the electrolyte,31,32 whereas almost no changes were observed for HCNTs–M2P (M = Co, Mn, Ni) electrode, suggesting the excellent methanol tolerance ability of HCNTs–M2P (M = Co, Mn, Ni). Fig. 7(d) reveals excellent stability of HCNTs–Co2P comparing with that of Pt/C. After continuous O2 reduction at −0.3 V (vs. Ag/AgCl) for 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, the HCNTs–Co2P electrode maintains about 92.5% of its initial current density, while the Pt/C electrode loses about 19%. The excellent methanol-resistance and stability can be ascribed to the characteristics of catalysts based on heteroatom-doped carbon.33,34
000 s, the HCNTs–Co2P electrode maintains about 92.5% of its initial current density, while the Pt/C electrode loses about 19%. The excellent methanol-resistance and stability can be ascribed to the characteristics of catalysts based on heteroatom-doped carbon.33,34
4. Conclusions
In this study, M2P (M = Co, Mn, Ni) nanoparticles decorated heteroatom-doped mesoporous carbon nanotubes were fabricated via the carbonization of transition metal precursors and polyphosphazene modified carbon nanotubes. Throughout the synthesis process, polyphosphazene played very important roles, for example, serving as surface modification agents and heteroatom precursors for carbon nanotubes, as well as proving phosphorus to form transition metal phosphides. Electrochemical tests manifested excellent ORR catalytic performance of HCNT–Mn2P and HCNT–Co2P in the aspect of high onset potential, high limited current density and 4e− dominated pathway. The electronic interaction between the embedded transition metal phosphide nanoparticles and heteroatom-doped carbon structures synergistically improved the ORR catalytic performance of the carbon nanotubes. This work provides a novel approach for rational design of high performance ORR catalysts based on heteroatom-doped carbon, as well as significant scientific basis for the design process.Acknowledgements
We gratefully acknowledge Natural Science Foundation of China (No. 21274092, 51133003, 61376003, 91441205), and Shanghai Science & Technology Committee (No. 10ZR1416100) for financial support.Notes and references
- J. Zhang, Z. Zhao, Z. Xia and L. Dai, Nat. Nanotechnol., 2015, 10, 444–452 CrossRef CAS PubMed.
- S. Gao, K. Geng, H. Liu, X. Wei, M. Zhang, P. Wang and J. Wang, Energy Environ. Sci., 2015, 8, 221–229 CAS.
- Z. Xiang, D. Cao, L. Huang, J. Shui, M. Wang and L. Dai, Adv. Mater., 2014, 26, 3315–3320 CrossRef CAS PubMed.
- W. Wei, H. Liang, K. Parvez, X. Zhuang, X. Feng and K. Müllen, Angew. Chem., 2014, 126, 1596–1600 CrossRef.
- D.-S. Yang, D. Bhattacharjya, M. Y. Song and J.-S. Yu, Carbon, 2014, 67, 736–743 CrossRef CAS.
- S. K. Ramasahayam, U. B. Nasini, V. Bairi, A. U. Shaikh and T. Viswanathan, RSC Adv., 2014, 4, 6306–6313 RSC.
- J. Chen, X. Wang, X. Cui, G. Yang and W. Zheng, Chem. Commun., 2014, 50, 557–559 RSC.
- C. You, S. Liao, H. Li, S. Hou, H. Peng, X. Zeng, F. Liu, R. Zheng, Z. Fu and Y. Li, Carbon, 2014, 69, 294–301 CrossRef CAS.
- J. Zhou, X. Shan, J. Ma, Y. Gu, Z. Qian, J. Chen and H. Feng, RSC Adv., 2014, 4, 5465–5468 RSC.
- Y. Zhang, T. Mori, J. Ye and M. Antonietti, J. Am. Chem. Soc., 2010, 132, 6294–6295 CrossRef CAS PubMed.
- L. Zhang and Z. Xia, J. Phys. Chem. C, 2011, 115, 11170–11176 CAS.
- Y. Zhao, L. Yang, S. Chen, X. Wang, Y. Ma, Q. Wu, Y. Jiang, W. Qian and Z. Hu, J. Am. Chem. Soc., 2013, 135, 1201–1204 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780–786 CrossRef CAS PubMed.
- Y. Liang, H. Wang, P. Diao, W. Chang, G. Hong, Y. Li, M. Gong, L. Xie, J. Zhou, J. Wang, T. Z. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2012, 134, 15849–15857 CrossRef CAS PubMed.
- Q. Li, S. Zhang, L. Dai and L.-S. Li, J. Am. Chem. Soc., 2012, 134, 18932–18935 CrossRef CAS PubMed.
- Y. Tan, C. Xu, G. Chen, X. Fang, N. Zheng and Q. Xie, Adv. Funct. Mater., 2012, 22, 4584–4591 CrossRef CAS.
- Y. Hu, J. O. Jensen, W. Zhang, L. N. Cleemann, W. Xing, N. J. Bjerrum and Q. Li, Angew. Chem., Int. Ed., 2014, 53, 3675–3679 CrossRef CAS PubMed.
- K. Chen, X. Huang, C. Wan and H. Liu, Chem. Commun., 2015, 51, 7891–7894 RSC.
- X. Zhang, X. Huang and X. Tang, J. Mater. Chem., 2009, 19, 3281–3285 RSC.
- J. Fu, Z. Chen, Q. Xu, J. Chen, X. Huang and X. Tang, Carbon, 2011, 49, 1037–1039 CrossRef CAS.
- Z. Liu, F. Peng, H. Wang, H. Yu, W. Zheng and X. Wei, J. Nat. Gas Chem., 2012, 21, 257–264 CrossRef CAS.
- G. Yang, H. Han, T. Li and C. Du, Carbon, 2012, 50, 3753–3765 CrossRef CAS.
- C. Wang, Y. Zhou, L. Sun, Q. Zhao, X. Zhang, P. Wan and J. Qiu, J. Phys. Chem. C, 2013, 117, 14912–14919 CAS.
- H. Liu, Y. Liu and D. Zhu, J. Mater. Chem., 2011, 21, 3335–3345 RSC.
- Z.-H. Sheng, L. Shao, J.-J. Chen, W.-J. Bao, F.-B. Wang and X.-H. Xia, ACS Nano, 2011, 5, 4350–4358 CrossRef CAS PubMed.
- D.-W. Wang and D. Su, Energy Environ. Sci., 2014, 7, 576–591 CAS.
- H. Hou, Q. Peng, S. Zhang, Q. Guo and Y. Xie, Eur. J. Inorg. Chem., 2005, 2625–2630 CrossRef CAS.
- D. Briggs and M. Seah, Practical Surface Analysis by Auger and X–ray Photoelectron Spectroscopy, John Wiley & Sons, New York, 1983 Search PubMed.
- Y. J. Sa, C. Park, H. Y. Jeong, S.-H. Park, Z. Lee, K. T. Kim, G.-G. Park and S. H. Joo, Angew. Chem., Int. Ed., 2014, 53, 4102–4106 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- V. A. Paganin, E. Sitta, T. Iwasita and W. Vielstich, J. Appl. Electrochem., 2005, 35, 1239–1243 CrossRef CAS.
- W. Liu and C.-Y. Wang, J. Electrochem. Soc., 2007, 154, B352–B361 CrossRef CAS.
- N. Daems, X. Sheng, I. F. J. Vankelecom and P. P. Pescarmona, J. Mater. Chem. A, 2014, 2, 4085–4110 CAS.
- D. W. Chang, H.-J. Choi and J.-B. Baek, J. Mater. Chem. A, 2015, 3, 7659–7665 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21385a |
| This journal is © The Royal Society of Chemistry 2015 |