Efficient lithium storage from modified vertically aligned carbon nanotubes with open-ends†
Abstract
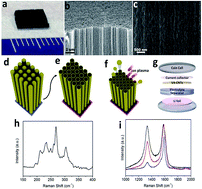
Herein, we report the use of vertically aligned carbon nanotubes (VA-CNTs) with controlled structure and morphology as an anode material for lithium-ion batteries. The tailored surface structure and open-end morphology of VA-CNTs made by ion milling and transfer processes increases the accessibility of Li ions, and allows Li ions to diffuse inside as well as on the surface of the CNTs through the generated surface defects, leading to the significantly improved lithium ion storage capacity compared to as-grown close-end VA-CNTs. The irreversible discharge capacity of the modified VA-CNTs anode reaches up to 2350 mA h g−1 at 2 C in the first cycle and the reversible capacity is in the range of 1200–557 mA h g−1 for the 2nd–20th cycles. The second insertion capacity of the modified CNTs electrode was 4 times higher than that of the as-grown CNTs and 3.2 times higher than that of a previously reported CNTs electrode for a commercial graphite device.

 Please wait while we load your content...
Please wait while we load your content...