Novel solid–solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage
Abstract
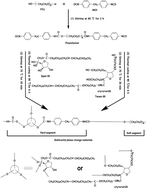
Polyurethane polymers were synthesized as novel solid–solid phase change materials (SSPCMs) by bulk polyaddition in the absence of organic solvents, in which polyethylene glycol (PEG) was selected as the working phase change substance and Span 80 and Tween 80 were used as crosslinking agents for the first time. The chemical structures, crystalline properties, phase change properties and thermal stability of the synthesized SSPCMs were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), differential scanning calorimetry (DSC) and thermogravimetric analysis (TG). An accelerated thermal cycling test was carried out to reveal the thermal reliability of the synthesized SSPCMs. The XRD patterns showed that the synthesized SSPCMs have a completely crystalline structure and defective crystallization compared with the pristine PEG. The DSC results showed that the synthesized SSPCMs have suitable phase change temperatures of 37–48 °C and high latent heats in the range of 120–130 J g−1. TG results showed that the synthesized SSPCMs possess good thermal stability.

 Please wait while we load your content...
Please wait while we load your content...