Reinforced chitosan beads by chitin nanofibers for the immobilization of β-glucosidase
Liang Liua,
Hechan Lva,
Jie Jianga,
Ke Zhenga,
Wenbo Yea,
Zhiguo Wang*b and
Yimin Fan*a
aJiangsu Key Lab of Biomass-based Green Fuel & Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing 210037, China. E-mail: fanyimin@njfu.edu.cn; Fax: +86 25 85427587; Tel: +86 25 85427587
bJiangsu Provincial Key Lab of Pulp and Paper Science and Technology, College of Light Industry Science and Engineering, Nanjing Forestry University, Nanjing 210037, China. E-mail: wzg@njfu.edu.cn; Tel: +86 25 85427118
First published on 15th October 2015
Abstract
A chitosan (CS) solution was filled with partially deacetylated α-chitin nanofibers (DEChN) and the composite (DEChN/CS) beads prepared thereof were used for the immobilization of β-glucosidase. The filling of chitin nanofibers resulted in an increase of the shear modulus (G′, G′′), which indicated an improvement to the mechanical properties of the DEChN/CS composite beads. Meanwhile, the specific surface area was improved from 46.47 m2 g−1 to 229.71 m2 g−1 when the chitosan beads were filled with the chitin nanofibers, and the prepared DEChN/CS composite beads had a broad pore size ranging from 20 nm to 60 nm. Moreover, a more porous and fibrous structure in the SEM image of the DEChN/CS beads was observed compared to that of the sole CS beads. The chitin nanofibers provided a fibrous network in the chitosan matrix that enhanced both the mechanical and porous properties of the DEChN/CS composite beads, which is normally considered contradictory. All of these enhanced features improved the efficiency of enzyme immobilization from 40% in the chitosan (CS) beads to 67% in the DEChN/CS composite beads.
Introduction
β-Glucosidase (β-D-glucoside glucohydrolases, cellobiase E.C.3.2.1.21) is a key enzyme in the hydrolysis of cellubiose to glucose, which are always involved in biomass degradation and ethanol production as a fuel from cellulosic agricultural residues.1 It can also be used in the synthesis of alkyl and aryl glycosides from natural polysaccharides or their derivatives, and the production of aromatic compounds, leading to the formation of products with applications in pharmaceutical, cosmetic, detergent and food industries, e.g. in the stabilization of juices and beverages, and in the improvement of the organoleptic properties of food and feed products. However, the poor operational stability and difficult reusability of free β-glucosidase, as well as its high production cost, have limited its large-scale industrial application.2 Therefore, improving the enzymatic hydrolysis efficiency has become a focus of current research.3 Enzyme immobilization was considered to be one of the ways to overcome the above shortcomings when the free enzyme was applied. Various techniques and carriers have been developed for enzyme immobilization, including adsorption, covalent linking to insoluble supports, entrapment in polymeric gels, encapsulation in membranes, crosslinking with bifunctional reagents (like glutaraldehyde), and different combinations of immobilization methods are also known.1Chitin and chitosan are natural polyaminosaccharides. Chitin is the second most abundant renewable natural structural polysaccharide after cellulose. Chemically, chitin is composed of β (1 → 4) linked 2-acetamido-2-deoxy-β-D-glucose units (or N-acetyl-D-glucosamine), forming a long chain linear polymer. It is insoluble in most solvents. Chitosan, the derivative of chitin, is obtained by N-deacetylation to a varying extent that is characterized by the degree of deacetylation, and is consequently a copolymer of N-acetyl-glucosamine and D-glucosamine. Chitosan is insoluble in water, but the presence of amino groups renders it soluble in acidic solutions below pH values of about 6.5.4 Chitosan has a great many significant biological and chemical properties.5 It is often used as enzyme immobilization supports in the form of powders, flakes and gels of different geometrical configurations. But, regardless of such interesting features, the usage of chitosan is usually limited due to their insufficient mechanical properties. Hence, their mechanical functionality is generally improved by blending with other polymers, such as collagen, silk, starch, and gelatin.6 Over the past several decades, nanofibers from chitin with appealing physical and biological features have attracted intense attention due to their excellent properties in relation to biodegradability, biocompatibility, antibacterial activity, low immunogenicity and wound healing capacity. And, in the last few decades, much attention has been paid to chitin/chitosan blended nanofibers regenerated from dissolved chitin and chitosan, which may enhance both the physical and biological functionality as it can take advantage of the favorable properties (strength/durability, enhancement of cell attachment) of both components. However, the processes have some drawbacks such as the fact that the solvents used are toxic, and the conditions used are harsh, which may do harm to enzyme immobilization.7
In this study, aqueous chitin nanofiber dispersions were blended with chitosan solutions; thereby, DEChN/CS composite beads were prepared in which the chitin nanofibers (DEChN) acted as the fillers, and were applied to the immobilization of the β-glucosidase. The filling of the chitin nanofibers had an effect on the properties of not only the DEChN/CS blends but also the composite beads, e.g. viscoelasticities, thermal curves, and morphologies. As a result, the enhanced DEChN/CS composite beads improved the enzyme immobilization efficiency.
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ml, and then the pH of the suspension was adjusted to about 3 using acetic acid under constant stirring. Then, this obtained suspension was homogenized and treated with ultrasonication, and after centrifugation, the chitin nano-dispersion was prepared successfully.
100 ml, and then the pH of the suspension was adjusted to about 3 using acetic acid under constant stirring. Then, this obtained suspension was homogenized and treated with ultrasonication, and after centrifugation, the chitin nano-dispersion was prepared successfully.The CS solution and DEChN dispersion were blended together in a beaker with constant stirring. The mass ratios of DEChN and CS were 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 and 1
7.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively, and the yielded blends were concentrated by heating at 90 °C to reach a total mass concentration of 2%. By using a syringe, the obtained blends were sprayed drop-wise at a constant rate into a neutralizing solution containing 5 M NaOH and 95% ethanol in a volume ratio of 5
5, respectively, and the yielded blends were concentrated by heating at 90 °C to reach a total mass concentration of 2%. By using a syringe, the obtained blends were sprayed drop-wise at a constant rate into a neutralizing solution containing 5 M NaOH and 95% ethanol in a volume ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The formed beads were left in solution for 12 h. Thereafter, the beads were washed with deionized water until the washing supernatant was neutral, and stored in deionized water at 4 °C. The composite beads prepared thereof were named as CS, DEChN(1)/CS(10), DEChN(1)/CS(7.5), and DEChN(1)/CS(5), respectively.
1. The formed beads were left in solution for 12 h. Thereafter, the beads were washed with deionized water until the washing supernatant was neutral, and stored in deionized water at 4 °C. The composite beads prepared thereof were named as CS, DEChN(1)/CS(10), DEChN(1)/CS(7.5), and DEChN(1)/CS(5), respectively.
The activity of the immobilized β-glucosidase was measured by the same procedure as described above. For each test, the three enzyme-loaded beads were added to 0.9 ml pNPG (5 mmol L−1) and allowed to react for 10 minutes in a water bath at 50 °C. The total immobilized enzyme activity was calculated according to the number of immobilized beads. The enzyme immobilization efficiency was expressed as a percentage of immobilized enzyme activity relative to the highest enzyme activity.
Instrumental analysis
Results and discussion
Characterization of the CS solution and the DEChN/CS blends
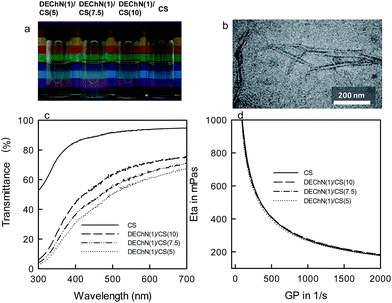
Fig. 1a shows photographs of the pure CS solution and the DEChN/CS blends of different ratios. The DEChN had widths of 6–7 nm and lengths of 200–800 nm, as shown in Fig. 1b. All of the solutions and the blends were transparent with high light transmittance (Fig. 1c) and stable to stand without any precipitation. Originally, the partially deacetylated chitin nanofibers (DEChN) could be dispersed in water at pH 3–4 to form stable and transparent aqueous nano-fiber dispersions. Here, instead of acidic water, the chitosan solution with a pH of around 3 might act as the dispersing media for chitin nanofibers to form the stable and transparent DEChN/CS blends. The phenomenon also indicated that there was no optical behavior typically caused by the aggregation of any bi- and multimolecular species within the tested concentration range and the interaction between CS and DEChN was not covalent grafting, but only simple mixing or physical blending.10,11The viscosities of the pure CS solution and the DEChN/CS blends with the same total concentration of 2% (w/v) are shown in Fig. 1d. All of the DEChN/CS blends had viscosities as high as that of the pure CS solution even with lower CS content. The viscosity of a polymer solution is a characteristic of its intermolecular interactions between polymer chains. The high viscosity of the chitosan solution is partly due to the strong hydrogen bonding between the NH2 and OH groups of chitosan polymer chains.1 However, within the DEChN/CS blends, the degree of deacetylation of DEChN was much lower than that of CS, and therefore with a lower average amount of NH2 groups, we came to a conjecture that the similar rheological properties of the DEChN/CS blends compared to those of the CS solution1 might be caused by the remarkably long lengths of the DEChN.
The similar UV-vis absorption spectra and the rheological properties of the CS solution and the DEChN/CS blends indicated that they might have similar fluid-to-gel (beads) processing properties.
Characterization of the CS and the DEChN/CS composite beads
The gel beads with an average diameter of 3 mm were successfully prepared from both the CS solution and the DEChN/CS blends, as shown in Fig. 2a. The FT-IR spectra, X-ray diffraction pattern and TG analysis were applied to characterize some of the physicochemical and thermal properties of the beads. | ||
| Fig. 2 Pictures (a), FT-IR (b), XRD (c) and TGA (d) of the CS beads and the DEChN/CS composite beads. | ||
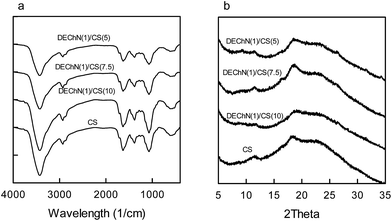
The spectra of all samples in the FT-IR spectra (Fig. 2b) included bands at approximately 3361, 2874, 1653, 1591, 1420, 1376, 1319, 1150, 1067, 1030, 897, 663 and 570 cm−1 that were related to the structure of chitosan.12 Though the spectra of the CS beads and the DEChN/CS composite beads were similar to each other as a whole, they still showed the differences in absorption intensities. As expected, the absorption bands of the amide I and amide II groups increased as the content of DEChN increased, while the peak of the amino groups (–NH2, 1591 cm−1) decreased correspondingly.13
X-ray diffraction patterns of the CS and the DEChN/CS beads are shown in Fig. 2c. The presence of the two peaks at 10.6° and 20° was in agreement with the characteristic diffractogram of the original chitosan.14 Compared to the spectra of CS, the peak at 9.4° in the spectra of DEChN/CS was similar to the spectra of chitin extracted from shrimp shell,15 which also indicated the presence of DEChN in the composite beads.
The thermal degradation of the CS and the DEChN/CS composite beads started at around 25 °C in a N2 atmosphere at a heating rate of 10 °C min−1 (Fig. 2d). All of the curves showed similar trends. The first stage, ranging between 50–100 °C might correspond to the loss of water, the second stage of weight loss started at 260 °C and continued up to 400 °C during which there was 50% weight loss due to the chemical degradation; the results corresponded well to the reported thermal degradation of the chitosan nanofibers.16,17 The filling of the small amount of chitin nanofibers did not affect the thermal degradability of the chitosan beads much.
Fig. 3 shows the G′ and G′′ modulus of the CS beads and the DEChN/CS composite beads at a frequency of 1 Hz (25 °C). For all beads, the loss modulus (G′′) was higher than the storage modulus (G′),18 which is a classic gel characteristic. It can be also observed that the samples with higher chitin nanofiber content (e.g. DEChN(1)/CS(5) and DEChN(1)/CS(7.5)) had a higher G′ and G′′ modulus, which indicated that the mechanical properties of the composite beads were improved by the filling of the DEChN.
 | ||
| Fig. 3 Rheological measurements of the storage modulus G′ and the loss modulus G′′ of the CS beads and the DEChN/CS composite beads. | ||
Morphology observations of the CS and the DEChN/CS composite beads
The morphology of the CS beads and the DEChN/CS composite beads were observed by SEM. Fig. 4a shows the surface morphology of the beads. In contrast to the sheetlike network with a few big pores on the surface of the CS beads, all of the DEChN/CS composite beads showed a fibrous network with a wealth of pores. According to the cross-section diagram shown in Fig. 4b, both the sheetlike structure and fibrous network were found in the CS beads, and as the percentage of the DEChN increased, less of the sheetlike section and a richer fibrous network appeared. Such structures promote the internal cross-linking and the porosity of the beads. Such a porous configuration indicated that the filling of the DEChN into the CS solution could lead to dramatic changes in the morphologic structure during the formation of the beads. | ||
| Fig. 4 Scanning electron micrographs of the CS beads and the DEChN/CS composite beads of the surface (a) and the cross-section (b) diagram. | ||
As shown in Fig. 5a, the CS and the DEChN/CS composite beads both exhibited type III nitrogen adsorption–desorption isotherms, the type III isotherm exhibited the prominent adsorption at high relative pressures (P/Po), indicating macropore adsorption. The BET specific surface areas for the CS, DEChN(1)/CS(10), DEChN(1)/CS(7.5) and DEChN(1)/CS(5) composite beads were 46.47 m2 g−1, 172.14 m2 g−1, 166.95 m2 g−1 and 229.71 m2 g−1, respectively. The greater the DEChN content, the larger the specific area of the beads, which indicated a decrease in the aggregation of the regenerated chitosan polymers by the filling of the chitin nanofibers. And the pore size distribution (Fig. 5b), calculated using the BJH method, clearly showed that compared to the CS beads, the DEChN/CS composite beads had a broader pore size ranging from 20–60 nm, which corresponded well to the normal enzyme size (ranging at the level of several hundreds of angstroms). The morphological characters and the pore size distribution of the DEChN/CS composite beads, together with the improved mechanical strength (indicated by the rheological measurements in Fig. 3), were supposed to be able to improve the enzyme loading, diffusing, and also enzyme immobilization.19,20
 | ||
| Fig. 5 Nitrogen adsorption/desorption isotherms (a) and pore size distribution (b) of the CS beads and the DEChN/CS composite beads. | ||
Immobilization of β-glucosidase
The crosslinking of glutaraldehyde was applied to the enzyme immobilization on the CS and DEChN/CS beads so as to further improve the immobilization efficiency.Fig. 6 shows the FT-IR and XRD spectra of the CS beads and the DEChN/CS composite beads after crosslinking by glutaraldehyde. All of the FT-IR spectra (Fig. 6a) showed the same peaks of 3423, 2929, 1629, 1382 and 1070 cm−1, which were different from the non-crosslinked ones (Fig. 2a). The XRD patterns also changed after crosslinking, as shown in Fig. 6b. Moreover, it was found that the numerical values of G′ and G′′ of the glutaraldehyde crosslinked CS and DEChN/CS beads (Fig. 7) were much higher than that of the non-crosslinked ones. All of the evidences indicated the effective crosslinking of glutaraldehyde with the CS and DEChN/CS beads. This crosslinking may not only promote the mechanical properties of the beads, but can also improve the immobilization efficiency of β-glucosidase.
 | ||
| Fig. 6 FT-IR (a) and XRD (b) spectra of the CS beads and the DEChN/CS composite beads after crosslinking with glutaraldehyde. | ||
 | ||
| Fig. 7 Rheological measurements of the storage modulus G′ and the loss modulus G′′ of the CS beads and the DEChN/CS composite beads after crosslinking with glutaraldehyde. | ||
Table 1 shows the immobilized and free enzyme activity of the β-glucosidase based on the different CS and DEChN/CS supports. The immobilized enzyme activity represents the amount of the enzyme immobilized on the CS and DEChN/CS composite beads, while the free enzyme activity represents the amount of the non-immobilized enzyme detected in the washing aqueous phase. As shown in Table 1 and Fig. 8, for all the beads, the total enzyme activities calculated by summarizing the immobilized and the free enzyme activities were quite close to the original added ones, which indicated the enzyme conservation during the immobilization process. And as obviously shown in Fig. 8, the CS beads showed the lowest immobilization efficiency (40%), while, as the percentage of the DEChN increased, the immobilization efficiency was promoted to as high as 67%.
| Samples (1 g) | Immobilized enzyme (IU g−1) | Free enzyme (IU g−1) | Total enzyme (IU g−1) |
|---|---|---|---|
| a The amount of enzyme (β-glucosidase) originally added was 39 IU g−1 B. | |||
| CS | 15.704 | 20.501 | 36.205 |
| DEChN(1)/CS(10) | 21.702 | 17.388 | 39.090 |
| DEChN(1)/CS(7.5) | 24.070 | 14.145 | 38.215 |
| DEChN(1)/CS(5) | 25.950 | 13.886 | 39.836 |
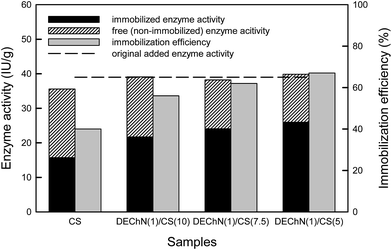 | ||
| Fig. 8 Comparison of enzyme immobilization efficiency on the CS beads and the DEChN/CS composite beads. | ||
Conclusions
Pure chitosan (CS) beads are commonly used as immobilization supports of enzymes or cells. In this study, chitin nano-fibers (DEChN) prepared from partially deacetylated α-chitins were firstly used as fillers to form DEChN/CS composite beads for application as enhanced immobilization supports.The partially deacetylated chitin nanofiber (DEChN) reinforced DEChN/CS composite beads exhibited a considerable improvement in the immobilization of β-glucosidase. The immobilization efficiency improved from 40% (CS) to 67% (DEChN(1)/CS (5)). The BET analysis, SEM observations and the rheological measurements of the CS and DEChN/CS composite beads showed that the DEChN/CS composite beads had higher porosity and better mechanical properties as well, which is normally considered contradictory. When compared with chitosan beads, the DEChN/CS composite beads not only kept their good biological properties, but also promoted the internal structure and the mechanical strength, which further improved the immobilization efficiency.
Acknowledgements
This research was supported by the National Forestry Public Welfare Industry Research Project (201304609), the National Natural Science Foundation of China (31100426), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (12KJA220001), the Specialized Research Fund for the Doctoral Program of the Higher Education of China (20133204110008) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- S. Ahmed, N. El-Shayeb, A. Hashem, S. Saleh and A. Abdel-Fattah, Braz. J. Chem. Eng., 2013, 30, 747–758 CrossRef CAS.
- T. Chen, W. Yang, Y. Guo, R. Yuan, L. Xu and Y. Yan, Enzyme Microb. Technol., 2014, 63, 50–57 CrossRef CAS.
- J. d. A. Figueira, F. F. G. Dias, H. H. Sato and P. Fernandes, Screening of supports for the Immobilization of Enzyme research, 2011 Search PubMed.
- B. Krajewska, Enzyme Microb. Technol., 2004, 35, 126–139 CrossRef CAS.
- B. Y. Sha, Q. S. Liu, J. L. Zhang and X. Y. Yin, Adv. Mater. Res., 2014, 887–888, 507–511 CrossRef CAS.
- F. Mirahmadi, M. Tafazzoli-Shadpour, M. A. Shokrgozar and S. Bonakdar, Mater. Sci. Eng., C, 2013, 33, 4786–4794 CrossRef CAS.
- F. Ding, H. Deng, Y. Du, X. Shi and Q. Wang, Nanoscale, 2014, 6, 9477–9493 RSC.
- Y. Fan, H. Fukuzumi, T. Saito and A. Isogai, Int. J. Biol. Macromol., 2012, 50, 69–76 CrossRef CAS.
- A. Brumbauer, G. Johansson and K. Réczey, . Chromatogr. B: Biomed. Sci. Appl., 2000, 743, 247–254 CrossRef CAS.
- K. Sugita, J. Yang and M. Shimojoh, Polym. Bull., 2008, 60(4), 449–455 CrossRef CAS.
- Z. Wu, W. Feng, Y. Feng, Q. Liu, X. Xu and T. Sekino, Carbon, 2007, 45, 1212–1218 CrossRef CAS.
- N. Bhattarai, D. Edmondson, O. Veiseh, F. A. Matsen and M. Zhang, Biomaterials, 2005, 26, 6176–6184 CrossRef CAS.
- E. S. Abdou, K. S. Nagy and M. Z. Elsabee, Bioresour. Technol., 2008, 99, 1359–1367 CrossRef CAS.
- I. Yamaguchi, S. Itoh, M. Suzuki, M. Sakane, A. Osaka and J. Tanaka, Biomaterials, 2003, 24, 2031–2036 CrossRef CAS.
- M. Hasegawa, A. Isogai and F. Onabe, Carbohydr. Polym., 1993, 20, 279–283 CrossRef CAS.
- G. Cárdenas, G. Cabrera, E. Taboada and S. P. Miranda, J. Appl. Polym. Sci., 2004, 93, 1876–1885 CrossRef.
- Y. S. Nam, W. H. Park, D. Ihm and S. M. Hudson, Carbohydr. Polym., 2010, 80, 291–295 CrossRef CAS.
- S. Kumar and J. Koh, Int. J. Biol. Macromol., 2012, 51, 1167–1171 CrossRef CAS.
- J. M. Zuidema, M. M. Pap, D. B. Jaroch, F. A. Morrison and R. J. Gilbert, Acta Biomater., 2011, 7, 1634–1643 CrossRef CAS.
- R. H.-Y. Chang, J. Jang and K. C.-W. Wu, Green Chem., 2011, 13, 2844–2850 RSC.
| This journal is © The Royal Society of Chemistry 2015 |