Side chain position, length and odd/even effects on the 2D self-assembly of mono-substituted anthraquinone derivatives at the liquid/solid interface†
Yi Hu,
Kai Miao,
Bao Zha,
Xinrui Miao,
Li Xu* and
Wenli Deng*
College of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: mslxu@scut.edu.cn; wldeng@scut.edu.cn; Tel: +86-20-22236708
First published on 16th October 2015
Abstract
The formation of self-assembled adlayers of 1-hydroxyanthraquinone (1-HA) and 2-hydroxyanthraquinone (2-HA) derivatives with various side chain length were investigated using scanning tunneling microscopy for the purpose of determining the influence of chemical structure on 2D molecular arrangement in a self-assembly process. Different structures labeled as Linear I, Linear II, Linear III, Linear IV and Z-like were presented based on their packing modes. Weak O⋯H–C hydrogen bonds existing between adjacent anthraquinone moieties are the key forces driving the formation of ribbon A, A′, B and C, which are the basic rows of the self-assembled structures. The emergence of odd or even numbers of carbon atoms in the alkyl chain inducing structural diversity is an indication that one of the driving forces for 1-HA-OCn (n = 15, 16) and 2-HA-OCn (n = 12, 14–16) molecules to assemble into ordered 2D nanostructures is the van der Waals interactions between interdigitated alkyl chains. 1-HA-OC16 and 1-HA-OC15 exhibited lamellar structures packed in Linear I and Linear II fashions. 2-HA-OC15 and 2-HA-OC16 adopted Linear III structures and Z-like packing modes. Moreover, when the number of carbon atoms in the side chain of 2-HA-OCn molecules was decreased to 12, the self-assembled pattern could present a Linear IV phase. Notably, 2-HA-OC14 showed the coexistence of Z-like and Linear IV phases. Systematic experiments revealed that a better understanding of the alkyl chain position, length and odd/even effects on 2D self-assembly would shed light on better control of assembly patterns and the design of new molecular materials.
Introduction
Massive research efforts are focusing on the regulation of the arrangement of organic molecules on surfaces for developing novel functional materials.1–5 Supramolecular two-dimensional (2D) structures on different substrates have attracted lots of attention for academic reasons as well as for their utility in wetting, lubrication, adhesion, adsorption and the fabrication of electronic and opto-electronic devices at the molecular scale.6–8 As a consequence, there is significant financial motivation to generate a more predictive understanding of 2D crystal polymorphism. A powerful method to investigate the 2D organization of organic molecules by physisorption on surfaces is scanning tunneling microscopy (STM) at the liquid/solid interface, which provides information on structure and dynamics, offering submolecular resolution of both periodic and nonperiodic packing in a time-dependent fashion.9–11 It is well-documented that successful monolayer formation and STM imaging require trimmed molecule–substrate, molecule–solvent and molecule–molecule interactions. Supramolecular structures are a result of the competition and balance between these interactions.12,13 The nature of functional groups, symmetrical characteristics, the number and position of the substituents, and the length of alkyl chains are the key factors in deciding the chemical structures of molecules, and thus play a decisive role in the formation of self-assembled nanostructures. A large quantity of research has reported the dependence of 2D supramolecular structures on alkyl chain length.14–16 Much attention has been attached to the influence of odd or even numbers of carbon atoms in side chains, which is a common phenomenon offering an alternate change of physical and chemical properties as well as the crystalline structure of a molecule.17–19 This side chain odd/even effect inducing different self-assembled structures can be obviously observed when the formation of the 2D arrangement is dominated by van der Waals interactions between the alkyl chains, and it emerges as a result of the end methyl groups aiming to minimize steric repulsion.17 Hydrogen bonds have been widely reported and explored for self-assembly purposes at the liquid/solid interface and in three-dimensional crystals due to the relatively strong, selective and directional nature of hydrogen bonding interactions.20 They can exert an influence on stabilizing self-assembled structures and weak O⋯H–C bonds have been widely reported in 2D crystal engineering.21,22Kikkawa et al. has intensively explored the self-assemblies of bipyridine derivatives on highly oriented pyrolytic graphite (HOPG), revealing fundamental insights into the role of the length, number and position of the substituents on molecular packing which is predominantly controlled by interactions between alkyl chains.17,23,24 Isomeric tetrathienoanthracene derivatives have been reported not long ago.25 The slight geometric difference between the two isomers means that the position of sulphur atoms in the molecule can lead to dramatic changes in the monolayer structures.
Herein, we investigated the supramolecular structures of a series of 1-hydroxyanthraquinone and 2-hydroxyanthraquinone derivatives (Scheme 1) with mono-substituent side chains at the liquid/solid HOPG interface with the help of STM. Through careful observation, different linear and Z-like structures were observed in the monolayers when the position or length of the alkyl chain changed. In one ribbon, molecules are gathered in a head-to-head fashion via weak O⋯H–C hydrogen bonds, and then all of the ribbons form a regular arrangement through alkyl chain interdigitation. In our research, the densely packed structures, which are entirely different from each other in their arrangement fashion, were compared and analysed in detail in order to explore how a slight chemical-structural change would influence the self-assembly process and result in a great difference in 2D molecular nanostructures.
The long alkyl chains show great commensurability with the graphite surface and are likely to arrange parallel to the substrate26 and meanwhile force the π-conjugated anthraquinone moieties to firmly adsorb on HOPG. High-resolution STM images can reveal the apparent features of the molecular packing fashion. We discovered that 1-HA-OC16 and 1-HA-OC15 showed totally different molecular arrangements, that is, Linear I and Linear II fashions, respectively. Not surprisingly, this arises from the different even and odd number of carbon atom in the alkyl chains. However, when the alkyl chain is in the 2-position, another two kinds of self-assembly structures, namely, Linear III and Z-like were generated for 2-HA-OC15 and 2-HA-OC16, respectively. Besides, we obtained another linear structure, called Linear IV, for 2-HA-OC12. 2-HA-OC14 adopted both Z-like and Linear IV phases, indicating that the alkyl chain length played an important role in the self-assembly process. These results can better promote our understanding of the effect of side chain position, length and odd or even properties on 2D molecular self-assembly.
Experimental section
1-Hydroxyanthraquinone (n = 15, 16) and 2-hydroxyanthraquinone (n = 12, 14–16) derivatives used in this study were synthesized as described in the ESI (Scheme S1†), and then recrystallized repeatedly (four to six times) in order to ensure a high degree of purity (≥98%). 1-octanoic acid solvent was purchased from Tokyo Chemical Industry without further purification. The solutions used in our work were at a concentration of 50% saturation. At first, 100% saturated solutions were prepared, and then they were diluted to 50% saturation according to accurate proportion. The samples were prepared by depositing a droplet (about 1 μL) of solution on a freshly cleaved atomically flat surface of HOPG (quality ZYB, Bruker, USA). STM measurements were performed on a Nanoscope IIIa Multimode SPM (Bruker, USA) under ambient conditions with the tip immersed in the supernatant liquid. The tips were mechanically cut from Pt/In wires (80/20). All images were recorded with a constant current mode and are shown without further processing. Tunneling parameters are given in the corresponding figure caption. Molecular models of the assembled structures were built using Materials Studio 4.4. The models were constructed based on the intermolecular distances and angles, and from the analysis of the STM results. Also, they were tested to be right according to the ideal overlap with the high-resolution STM images. The DSC experiments were conducted with a scan rate of 10 °C min−1 for heating and cooling (instrument: NETZSCH DSC 200F3). Room temperature and humidity were recorded to be 15–20 °C and 45–55%.Results
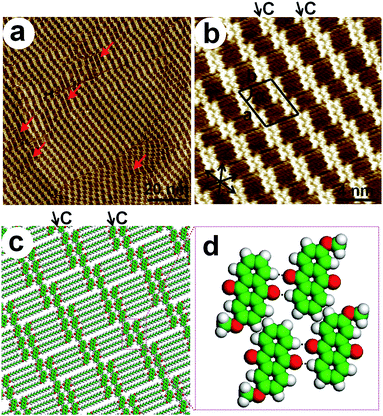
1-HA-OCn and 2-HA-OCn molecules, consisting of π-conjugated anthraquinone moieties and alkyl chains, are expected to generate distinguishable 2D nanostructures at the liquid/solid interface. An anthraquinone core substituted by a single alkyl chain in the 1-position and 2-position adopt completely different self-assembly patterns, which are constituted from four basic ribbon structures defined as ribbon A, A′, B and C, as shown in Fig. 1. All of the aggregation modes illustrate that the anthraquinone moieties stack with a regular sequence via a head-to-head fashion. For ribbon A and A′, the anthraquinone portions arrange in a V-like shape, but during our experiments, careful observation proves that they are not mirror images. Ribbon A′ is the same with ribbon A as the result of a rotation of 180°, but they exhibit different ribbon orientation in phases consisting of both of them. Nevertheless, in the case of ribbon B, the two rows of π-conjugated units are antiparallel to each other. Compared with ribbon B, ribbon C shows regular dislocation of the antiparallel dimers, resulting in alternately aligned tetramers and hexamers. | ||
| Fig. 1 Four different aggregation modes of ribbon A, A′, B and C. The anthraquinone cores and side chains are represented with green shapes and black lines, respectively. | ||
In our present research, different kinds of 2D self-assembled networks were observed at the liquid/HOPG interface with 1-octanoic acid as the solvent under the same concentration of 50% saturation. No solvent coadsorption was observed, indicating that 1-octanoic acid exerted its function only as a dispersant.27
Self-assembly of 1-HA-OC16
Firstly, the 2D self-assembly of 1-HA-OC16 was explored. 1-HA-OC16 forms a linear structure on the HOPG surface, exhibiting a lamellar fashion (Fig. 2 and S1†), named Linear I, which is constituted from ribbon A′. Fig. 2a is the high-resolution STM image, showing the details of this self-assembled structure. The brighter rods and darker troughs represent anthraquinone moieties and alkyl chains, respectively, ascribing to higher electronic density for the former and lower for the latter.27 It is obvious that all of the ribbons have the same orientation, and every ribbon consists of two rows of molecules which are packed in a head-to-head and V-like mode. A set of arrows in the left bottom of Fig. 2a is depicted, showing the main symmetry of HOPG under the monolayer. 1-HA-OC16 molecules arrange parallel to the substrate, with the alkyl chains extending along the main direction of the substrate. According to the molecular arrangement, a structural model is proposed in Fig. 2b, corresponding well with the experimentally obtained STM images. Careful observation indicates that the ribbon is not mirror-symmetric. For this linear packing fashion, alkyl chains are supposed to not be fully interdigitated, which means that fifteen carbon atoms in a chain participate in the interdigitation between neighboring side chains, as shown in Fig. S2.† This dislocation by one atom is caused by the requirement for minimum steric repulsion, which is to be discussed in this paper. In this V-like ribbon, two rows of molecules are staggered tightly, through weak hydrogen bonds O⋯H–C in adjacent anthraquinone cores, as indicated in Fig. 2c. The unit cell parameters for this Linear I arrangement can be defined as a = 0.8 ± 0.1 nm, b = 3.0 ± 0.1 nm and α = 86 ± 1°. Every unit cell consists of two molecules and the calculated area density is 1.20 nm2 per molecule.Self-assembly of 1-HA-OC15
For the purpose of exploring how odd and even numbers of carbon atoms in the side chain can influence the 2D self-assembly process, we decreased the alkyl chain length of 1-HA-OCn to n = 15. Fig. 3a shows the high-resolution STM image of 1-HA-OC15 molecules adsorbed on the HOPG surface. In the large-scale STM image of 1-HA-OC15 (shown in Fig. S3†), the HOPG surface is covered with an ordered molecular adlayer, named as Linear II, which is constituted from the basic ribbon of B. The ribbon is composed of two molecular rows, which take a head-to-head and antiparallel configuration. The alkyl chains are arranged in an interdigitated way, leading to close packing. The black arrows indicating the lattice direction of the graphite are superimposed in Fig. 3a. The alkyl chains extend along the main direction of the substrate flatly, showing great compatibility with the graphite lattice, and thus make the 1-HA-OC15 molecules tightly adsorb on the substrate surface through van der Waals interactions. A proposed structural model for this linear arrangement is depicted in Fig. 3b and it is in good agreement with the STM results. Carbon atoms of neighboring chains tend to be arranged with each “up” atom in one chain next to a “down” atom in each of the two neighbors. This condition makes us come up with a guess that only fourteen carbon atoms in the alkyl chains of 1-HA-OC15 are alternated, instead of an entirely alternated chain (as shown in Fig. S4†). That is to say, a highly matched structure can be achieved for 1-HA-OC15 molecules if the end methyl and the second methylene which is connected to the ether group in the adjacent two chains are pointing in the same direction. In order to minimize the steric repulsion, this shift by one atom along the side chain is reasonable and it will be systematically discussed in the Discussion section. The weak hydrogen bonds O⋯H–C between two rows of anthraquinone moieties in a ribbon are illustrated in Fig. 3c. A unit cell for this linear structure is outlined with the parameters of a = 1.0 ± 0.2 nm, b = 3.3 ± 0.2 nm and α = 84 ± 2°. Every unit cell consists of two molecules and the calculated area density is 1.64 nm2 per molecule.Self-assembly of 2-HA-OC15
In the case of the 2-HA-OCn derivatives, different linear structures were observed, which is powerful evidence for the importance of the substituent position on the molecular self-assembly process. Fig. 4a is the large-scale STM image for 2-HA-OC15 on the HOPG surface. Except for some disconnected single rows, as marked by red arrows, which occur by chance, the adlayer consists of three kinds of ribbons, ribbon A, A′ and B. This linear arrangement, defined as Linear III, can also be considered as a combination of two domains: one domain made up from ribbon A and another made up from ribbon A′. These domains are orienting in reverse directions, as noted by pink and green arrows for ease of distinction. Ribbon B is seldom found and it is just a needed interim ribbon, guaranteeing smooth transition between the reverse domains, instead of the occurrence of unstable domain boundaries. A set of black arrows in Fig. 4b indicates the lattice direction of the graphite, and the alkyl chains extend along the symmetry axis of the substrate. The alkyl chains are fully interdigitated with each other through van der Waals interactions and two rows of molecules in a ribbon are staggered via hydrogen bonds. The unit cell parameters for this linear arrangement are a = 1.1 ± 0.3 nm, b = 3.0 ± 0.3 nm and α = 88 ± 3°. Every unit cell consists of two molecules and the calculated area density is 1.65 nm2 per molecule. Fig. 4d is an illustration of weak hydrogen bonds O⋯H–C between two rows of the ribbons.Self-assembly of 2-HA-OC16
Odd or even numbers of carbon atoms in side chains can immensely affect the self-assembly structure, and this phenomenon has also been found when the substituent group is in the 2-position. 2-HA-OC16 molecules form a Z-like adlayer, named Z-like, in which the anthraquinone moieties arrange in a zigzag shape. The basic ribbon is ribbon C. Fig. 5a is the STM image of a large area. Several obvious single rows indicated by red arrows, which appear randomly and destroy the uniformity of the adlayer, can be regarded as accidental occurrences. Fig. 5b is the high-resolution STM image of 2-HA-OC16, showing detailed packing information for this non-straight linear structure. The black arrows in the bottom left of Fig. 5b indicate the main symmetry of the HOPG. The long alkyl chains show good commensurability with the graphite surface in order to maximize the adsorbate–substrate interaction. On the basis of the STM results, a structural model for this Z-like packing fashion is proposed, as shown in Fig. 5c, which matches well with the observed molecules. A unit cell is presented in Fig. 5b with a = 4.6 ± 0.2 nm, b = 3.7 ± 0.2 nm and α = 73 ± 1°. The unit cell consists of ten molecules and the calculated area density is 1.63 nm2 per molecule. This Z-like arrangement is similar to the Linear II structure for 1-HA-OC15, that is to say, the former can be regarded as the result of a one-molecule-dislocation from the latter for every two or three dimers, along the alkyl chain direction. As a result, tetramers and hexamers are formed. Careful observation discovered that there is no disciplinary emergence for tetramers and hexamers in a Z-like ribbon, for instance, a tetramer–hexamer–tetramer–hexamer arrangement, but the adjacent ribbons arrange in a perfectly dense packing fashion. The weak hydrogen bonds O⋯H–C in tetramer and hexamer aggregations are illustrated in Fig. 5d.Self-assembly of 2-HA-OC12 and 2-HA-OC14
As a step further, we decreased the alkyl chain length of 2-HA-OCn to n = 12 and 14, with the purpose of understanding the effect of chain length on self-assembly. Another linear structure labeled as Linear IV was observed. From a large-scale STM image for 2-HA-OC12 (Fig. S5†), it can be clearly seen that the 100 × 100 nm2 area is not uniform, which consists of several domains with distinct boundaries. Fig. 6a is the high-resolution STM image, exhibiting careful assembly information. The Linear IV arrangement is constituted by regularly staggered V-like ribbons of A and A′. The unit cell parameters are a = 0.8 ± 0.3 nm, b = 5.9 ± 0.3 nm and α = 89 ± 2°. Every unit cell consists of four molecules and the calculated area density is 1.18 nm2 per molecule. The alkyl chains all extend along the same direction (the lattice direction of the graphite, the black arrows as shown in Fig. 6a), are parallel to each other and are fully interdigitated. On the basis of a large amount of high-resolution STM images, a structural model for this Linear IV packing fashion is proposed in Fig. 6c, which is in good agreement with the experimental results. V-like ribbons of A and A′ are reversed to each other but with the same visual appearance. Two rows of molecules are arranged regularly through hydrogen bonds, which are illustrated in Fig. 6d. Interestingly, 2-HA-OC14 molecules self-assemble into a monolayer consisting of both Linear IV and Z-like phases, as shown in Fig. 6b and S6.† The basic unit cells are imposed in Fig. S6b and S6c† with the parameters of a = 0.9 ± 0.1 nm, b = 6.3 ± 0.1 nm, α = 84 ± 1° for the Linear IV structure and a = 5.5 ± 0.2 nm, b = 3.6 ± 0.2 nm, α = 67 ± 2° for the Z-like structure. According to the unit cell parameters, the calculated area density are greatly different, namely, 1.41 nm2 per molecule for the Linear IV pattern and 1.82 nm2 per molecule for the Z-like network. We can then conclude that the Linear IV structure is denser than the Z-like structure. That is to say, as the alkyl chain length decreases, 2-HA-OCn molecules form Z-like (n = 16), Z-like and Linear IV (n = 14), and Linear IV (n = 12) phases, which can be attributed to the chain-length effect.Discussion
Table 1 systematically summarizes the linear and Z-like structures from several aspects of the basic packing fashion, shape, unit cell parameters, geometric characteristics and molecular density. The monolayer morphology is governed by the chemical structure, and both 1-HA-OCn and 2-HA-OCn molecules showed a great dependence on the side chain position, length and odd/even effect. Therefore, the 2D self-assembly of anthraquinone derivatives can be tuned by efficiently changing the properties of the alkyl chains. For a better understanding of these relationships and easy comparison of Linear I, Linear II, Linear III, Linear IV and Z-like configurations, we made a graphical summary of the structural model, as shown in Fig. 7.| Molecule | Structure | Basic ribbon | Ribbon shape | Unit cell parameters | N a | S (nm2) b | ||
|---|---|---|---|---|---|---|---|---|
| a (nm) | b (nm) | α (°) | ||||||
| a N = number of molecules per unit cell.b S represents the area density. | ||||||||
| 1-HA-OC16 | Linear I | A′ | V-like | 0.8 ± 0.1 | 3.0 ± 0.1 | 86 ± 1 | 2 | 1.20 |
| 1-HA-OC15 | Linear II | B | Antiparallel | 1.0 ± 0.2 | 3.3 ± 0.2 | 84 ± 2 | 2 | 1.64 |
| 2-HA-OC15 | Linear III | A, A′, B | V-like and antiparallel | 1.1 ± 0.3 | 3.0 ± 0.3 | 88 ± 3 | 2 | 1.65 |
| 2-HA-OC16 | Z-like | C | Antiparallel | 4.6 ± 0.2 | 3.7 ± 0.2 | 73 ± 1 | 10 | 1.63 |
| 2-HA-OC14 | Z-like | C | Antiparallel | 5.5 ± 0.2 | 3.6 ± 0.2 | 67 ± 2 | 10 | 1.82 |
| Linear IV | A, A′ | V-like | 0.9 ± 0.1 | 6.3 ± 0.1 | 84 ± 1 | 4 | 1.41 | |
| 2-HA-OC12 | Linear IV | A, A′ | V-like | 0.8 ± 0.3 | 5.9 ± 0.3 | 89 ± 2 | 8 | 1.18 |
On the basis of the STM analysis, the 2D structure formation for the 1-HA-OCn and 2-HA-OCn derivatives is dominated by (i) hydrogen bonds of the anthraquinone units, (ii) intermolecular van der Waals interactions of the alkyl chains, and (iii) molecule–substrate interactions. Adjacent molecules in ribbon A, A′, B and C are staggered via weak hydrogen bonds between π-conjugated anthraquinone moieties (i). Ribbons are arranged mutually by alkyl chain interdigitation (ii). The monolayer adsorbed tightly on the HOPG surface by the interplay between the adsorbate and substrate (iii).
Weak hydrogen bonding interactions between anthraquinone units
Weak hydrogen bonds can direct the formation of 2D crystalline structures28 and molecular recognition processes, ascribing to their high directionality. For 1-HA-OCn and 2-HA-OCn derivatives, weak O⋯H–C hydrogen bonds are found, with the carbonyl oxygen as an acceptor and hydrogen in the adjacent anthraquinone moieties as a donor. This type of hydrogen bonding has been reported before.21,22 Moreover, similar weak hydrogen bonds formed between anthraquinone units have been reported by Tamaki et al.29 A hydrogen atom in the benzene ring has the ability to form O⋯H–C hydrogen bonds,30,31 which will, as a consequence, significantly impact 2D self-assembly formation.In our research, the π-conjugated anthraquinone units in ribbon A, A′, B and C are arranged in two fashions, V-like and antiparallel patterns, and they gathered with each other not only through common van der Waals interactions, but also via weak hydrogen bonds, as shown in Fig. 2c, 3c, 4d, 5d and 6d. It is well known that molecules under given ambient conditions favor assembled structures which are as dense as possible. Upon comparing the molecule density of 1-HA-OC15 and 2-HA-OC15, we found that their compact degree values were nearly the same, namely, 1.64 nm2 per molecule for 1-HA-OC15 and 1.65 nm2 per molecule for 2-HA-OC15 (as the S value indicated in Table 1). As a consequence, we conclude that close-packed structures can be obtained whether through weak hydrogen bonds induced V-like or antiparallel combination modes. However, 2-HA-OC16 showed lower molecule density (1.63 nm2 per molecule) than 1-HA-OC16 (1.20 nm2 per molecule), indicating that dimer dislocation in a ribbon along the direction of the alkyl chains would cause the decrease of compact degree.
Side chain position-induced different self-assemblies
Chemical structure is one of the crucial factors dominating the formation of 2D molecular self-assembly networks on solid substrates and thus can be utilized for the purpose of desirable surface patterns.27,32–34 The change of the alkyl chain position from position 1 to 2 resulted in tremendous variation of physical properties as well as for self-assembled structures. Fig. 8a shows the differential scanning calorimetry (DSC) thermograms of 1-HA-OCn (n = 15, 16) and 2-HA-OCn (n = 12, 14–16). In addition, a series of 1-HA-OCn and 2-HA-OCn (n = 11 to 18) compounds were synthesized in order to get enough DSC data and then summarize the transformation regularity as the position, length and odd or even number of atoms of the side chain change (Fig. S7†). 1-HA and 2-HA derivatives show a gradually changed phase-transition temperature (obtained from differential scanning calorimetry testing, on the trace of heating), which are assigned to the melting point. In Fig. 8b, the blue line is above the red line, demonstrating that the melting point for 1-HA-OCn is higher than that for 2-HA-OCn. This significant increase of phase-transition temperature can be ascribed to the relatively large energy required to form an isotropous and clear phase from the solid state under ambient conditions.35,36 Therefore, we can conclude that 1-HA-OCn and 2-HA-OCn derivatives need quite different energy for self-assembling into an ordered 2D formation, thus different kinds of structures are formed, which are stable both thermodynamically and kinetically.Effect of odd or even number of atoms of the side chain on 2D self-assembly
Whether the number of carbon atoms in the side chains is odd or even has also been well-known to affect the properties of organic molecules and their packing fashions at liquid/solid interface. It can be evidently seen that the melting point of both 1-HA-OCn and 2-HA-OCn derivatives increase in an up-down-up-down tendency, as the zigzag lines demonstrate, corresponding to the odd-even-odd-even number of the carbon atoms (Fig. 8b). The structural difference induced by odd or even numbers of carbon atoms in the alkyl chain is proposed as a result of the requirement for the end methyl group to minimize steric repulsion, and its appearance to the system is an indication that the major driving force of the 2D structural formation is the intermolecular interplay of alkyl chains. 1-HA-OC15 and 1-HA-OC16, or 2-HA-OC15 and 2-HA-OC16 adopted distinguishing assembled patterns, owing to making the van der Waals interactions between the side chains reach the maximum comfort and energetic equilibrium. Carbon atoms of neighboring chains should be staggered, with each “up” atom in one chain next to a “down” atom in each of the two neighbors, in order to get dense 2D packing on the surface.37 Therefore, this condition can be achieved by (1) atom shift, resulting in partially interdigitated alkyl chains, for example, Linear I and Linear II packing fashions. (2) Molecular rotation of 180°, resulting in opposite ribbon directions, such as in the Linear IV phase.Even though STM cannot observe the interdigitated alkyl chains to the high accuracy of single atoms, we predict that the side chains of 1-HA-OC15 and 1-HA-OC16 molecules are partially interdigitated. Fig. 9 shows the space filling of the matched methyl and methylene groups in the side chains with pink arrows indicating the “down” direction and blue arrows indicating the “up” direction. Orange and yellow rectangles represent the repulsive and matched carbon atoms in the neighboring side chains, respectively. Fully interdigitated alkyl chains are favored in the consideration of close packing. However, in our research, we guessed that partially interdigitated ones are more reasonable for 1-HA-OC15 and 1-HA-OC16 molecules. In Linear I packing fashion for 1-HA-OC16, if alkyl chains are fully interdigitated, as shown in Fig. 9a, the adjacent carbon atoms in neighboring side chains will point in the reverse direction, and this repulsive condition can result in great steric repulsion. However, in the case of partially interdigitated conditions as shown in Fig. 9b, which can be achieved by one atom shift, alkyl chains are highly matched and these conditions are more reasonable. Therefore, we assume that only fifteen carbon atoms in the alkyl chains of 1-HA-OC16 are alternated. Similarly, for the Linear II packing fashion of 1-HA-OC15, fourteen carbon atoms in a chain participate in the interdigitation between neighboring side chains (Fig. 9d), instead of fully interdigitated one (Fig. 9c). This shift by one atom is also caused by the requirement for minimum steric repulsion. Our STM results indicate that in the Linear IV packing fashion for 2-HA-OC12, the adjacent V-like ribbons point in the opposite direction (as shown in Fig. 9f), and as a result, carbon atoms of neighboring chains are staggered, with each “up” atom in one chain matching well with the next “down” atom in each of the two neighbors. Compared with another condition we assumed in Fig. 9e, in which ribbons of 2-HA-OC12 are pointing in the same direction and then neighboring carbon atoms are repulsive to each other, we affirm that this rotation of 180° is needed for the purpose of minimizing steric repulsion. Besides, in the case of the Linear III structure for 2-HA-OC15 (Fig. 9g) and Z-like structure for 2-HA-OC16 (Fig. 9h), no atom shift or molecule rotation are needed, because these kinds of fully interdigitated fashions achieve ideally dense packing and minimum steric repulsion.
Side chain length-induced structural difference for 2-HA-OCn (n = 12, 14, 16)
The dependence of 2D supramolecular structures on alkyl chain length is a common phenomenon. On the basis of STM observation, we found that 2-HA-OCn derivatives displayed apparent structural differences when the alkyl chain is shortened from 16 to 12. 2-HA-OC16 favored a Z-like packing fashion, 2-HA-OC12 adopted the Linear IV 2D nanopattern, but 2-HA-OC14 displayed a configuration of coexistent Z-like and Linear phases (see Fig. 6b, S6b and S6c†). Then the question arises as to why the gradually decreased alkyl chain length can lead to related structural change. According to the unit cell parameters shown in Table 1, the calculated area density for Linear IV and Z-like patterns of 2-HA-OC14 are greatly different, namely, 1.41 nm2 per molecule for Linear IV and 1.82 nm2 per molecule for Z-like. We then can conclude that the Linear IV structure is denser than the Z-like structure. It is well-known that the 2D self-assembled structures at the liquid/solid interface involve a balance and competition between adsorption and desorption of the solute molecules.38 The adsorption of molecules from the solution is under kinetic control.39 As the CH2 units in the side chains decrease, the van der Waals interactions between the solvent and solute molecules decrease. Therefore, the number of molecules adsorbed from the solution onto the surface per area and time increase monotonically with the decrease of side chain length,21 and thus favors the formation of Linear IV with denser area density.Conclusion
STM observations of 1-HA-OCn and 2-HA-OCn molecules were performed at the liquid/solid interface. Different linear and Z-like self-assembly patterns called Linear I, Linear II, Linear III, Linear IV and Z-like were found, by changing the chemical structures. Two molecular rows in each ribbon aggregated with each other via weak O⋯H–C hydrogen bonds between π-conjugated anthraquinone moieties and formed head-to-head arrangement modes. In the lamellae, ribbons gathered together via alkyl chain interdigitation. Careful analysis of the experimental unit cell parameters for 1-HA-OC15 and 2-HA-OC15 allows us to draw a conclusion that V-like and antiparallel combination styles for anthraquinone moieties have similar packing density, while dislocation which may result in Z-like ribbon can lead to less dense molecular arrangement. Linear I and Linear II, Linear III and Z-like patterns were induced by the odd/even properties of the side chains. Linear I and Z-like, Linear II and Linear III structures were different, ascribed to different side chain position. Besides, the difference between Z-like and Linear IV configurations was caused by alkyl chain length. These results show that position of the substituent, length of the alkyl chain and the odd or even number of carbon atoms in the side chain can immensely affect the packing fashion of different adlayers, and thus should be taken into account when regulating 2D molecular self-assembly nanostructures.Acknowledgements
Financial support from the National Program on Key Basic Research Project (2012CB932900), the National Natural Science Foundation of China (51373055, 21403072, 21573077), the China Postdoctoral Science Foundation (2014M552189), and the Fundamental Research Funds for the Central Universities (SCUT) are gratefully acknowledged.Notes and references
- C. Marie, F. Silly, L. Tortech, K. Müllen and D. Fichou, ACS Nano, 2010, 4, 1288–1292 CrossRef CAS PubMed.
- J. A. Elemans, I. de Cat, H. Xu and S. de Feyter, Chem. Soc. Rev., 2009, 38, 722–736 RSC.
- L. Piot, F. Silly, L. Tortech, Y. Nicolas, P. Blanchard, J. Roncali and D. Fichou, J. Am. Chem. Soc., 2009, 131, 12864–12865 CrossRef CAS PubMed.
- J. F. Dienstmaier, K. Mahata, H. Walch, W. M. Heckl, M. Schmittel and M. Lackinger, Langmuir, 2010, 26, 10708–10716 CrossRef CAS PubMed.
- F. P. Cometto, K. Kern and M. Lingenfelder, ACS Nano, 2015, 9, 5544–5550 CrossRef CAS PubMed.
- F. Tao and S. L. Bernasek, Chem. Rev., 2007, 107, 1408–1453 CrossRef CAS PubMed.
- H. B. Fang, L. C. Giancarlo and G. W. Flynn, J. Phys. Chem. B, 1998, 102, 7421–7424 CrossRef CAS.
- D. M. Cyr, B. Venkataraman and G. W. Flynn, Chem. Mater., 1996, 8, 1600–1615 CrossRef CAS.
- X. Y. Yang, X. Dou, A. Rouhanipour, L. J. Zhi, H. J. Räder and K. Müllen, J. Am. Chem. Soc., 2008, 130, 4216–4217 CrossRef CAS PubMed.
- H. Zhou, H. Dang, J. H. Yi, A. Nanci, A. Rochefort and J. D. Wuest, J. Am. Chem. Soc., 2007, 129, 13774–13775 CrossRef CAS PubMed.
- S. S. Li, H. J. Yan, L. J. Wan, H. B. Yang, B. H. Northrop and P. J. Stang, J. Am. Chem. Soc., 2007, 129, 9268–9269 CrossRef CAS PubMed.
- F. Tao and S. L. Bernasek, Langmuir, 2007, 23, 3513–3522 CrossRef CAS PubMed.
- S. de Feyter and F. C. de Schryver, J. Phys. Chem. B, 2005, 109, 4290–4302 CrossRef CAS PubMed.
- Y. Miyake, T. Nagata, H. Tanaka, M. Yamazaki, M. Ohta, R. Kokawa and T. Ogawa, ACS Nano, 2012, 6, 3876–3887 CrossRef CAS PubMed.
- X. Shao, X. C. Luo, X. Q. Hu and K. Wu, J. Phys. Chem. B, 2006, 110, 15393–15402 CrossRef CAS PubMed.
- K. Tahara, K. Inukai, N. Hara, C. A. Johnson II, M. M. Haley and Y. Tobe, Chem.–Eur. J., 2010, 16, 8319–8328 CrossRef CAS PubMed.
- Y. Kikkawa, E. Koyama, S. Tsuzuki, K. Fujiwara and M. Kanesato, Langmuir, 2010, 26, 3376–3381 CrossRef CAS PubMed.
- F. Tao, J. Goswami and S. L. Bernasek, J. Phys. Chem. B, 2006, 110, 4199–4206 CrossRef CAS PubMed.
- L. Xu, X. R. Miao, B. Zha, K. Miao and W. L. Deng, J. Phys. Chem. C, 2013, 117, 12707–12714 CAS.
- K. S. Mali, K. Lava, K. Binnemans and S. de Feyter, Chem.–Eur. J., 2010, 16, 14447–14458 CrossRef CAS PubMed.
- L. Kampschulte, M. Lackinger, A. K. Maier, R. S. K. Kishore, S. Griessl, M. Schmittel and W. M. Heckl, J. Phys. Chem. B, 2006, 110, 10829–10836 CrossRef CAS PubMed.
- L. Kampschulte, T. L. Werblowsky, R. S. K. Kishore, M. Schmittel, W. M. Heckl and M. Lackinger, J. Am. Chem. Soc., 2008, 130, 8502–8507 CrossRef CAS PubMed.
- Y. Kikkawa, E. Koyama, S. Tsuzuki, K. Fujiwara, K. Miyake, H. Tokuhisa and M. Kanesato, Surf. Sci., 2007, 601, 2520–2524 CrossRef CAS.
- Y. Kikkawa, E. Koyama, S. Tsuzuki, K. Fujiwara, K. Miyake, H. Tokuhisa and M. Kanesato, Chem. Commun., 2007, 1343–1345 RSC.
- C. Fu, F. Rosei and D. F. Perepichka, ACS Nano, 2012, 6, 7973–7980 CrossRef CAS PubMed.
- J. P. Rabe and S. Buchholz, Science, 1991, 253, 424–427 CAS.
- X. Zhang, Q. Chen, G. J. Deng, Q. H. Fan and L. J. Wan, J. Phys. Chem. C, 2009, 113, 16193–16198 CAS.
- A. Ciesielski, C. A. Palma, M. Bonini and P. Samori, Adv. Mater., 2010, 22, 3506–3520 CrossRef CAS PubMed.
- T. Yoshinori, M. Kosuke and M. Kazuo, Bull. Chem. Soc. Jpn., 2013, 86, 354–362 CrossRef.
- C. Meier, U. Ziener, K. Landfester and P. Weihrich, J. Phys. Chem. B, 2005, 109, 21015–21027 CrossRef CAS PubMed.
- Z. C. Mu, L. J. Shu, H. Fuchs, M. Mayor and L. F. Chi, J. Am. Chem. Soc., 2008, 130, 10840–10841 CrossRef CAS PubMed.
- L. J. Wan, Acc. Chem. Res., 2006, 39, 334–342 CrossRef CAS PubMed.
- J. V. Barth, Annu. Rev. Phys. Chem., 2007, 58, 375–407 CrossRef CAS PubMed.
- S. S. Li, B. H. Northrop, Q. H. Yuan, L. J. Wan and P. J. Stang, Acc. Chem. Res., 2009, 42, 249–259 CrossRef CAS PubMed.
- J. Y. Wang, J. Yan, L. Ding, Y. Ma and J. Pei, Adv. Funct. Mater., 2009, 19, 1746–1752 CrossRef CAS.
- L. Y. Park, D. G. Hamilton, E. A. McGehee and K. A. McMenimen, J. Am. Chem. Soc., 2003, 125, 10586–10590 CrossRef CAS PubMed.
- K. G. Nath, O. Ivasenko, J. A. Miwa, H. Dang, J. D. Wuest, A. Nanci, D. F. Perepichka and F. Rosei, J. Am. Chem. Soc., 2006, 128, 4212–4213 CrossRef CAS PubMed.
- Y. B. Li, Z. Ma, G. C. Qi, Y. L. Yang, Q. D. Zeng, X. L. Fan, C. Wang and W. Huang, J. Phys. Chem. C, 2008, 112, 8649–8653 CAS.
- L. Xu, X. R. Miao, L. H. Cui, P. Liu, X. F. Chen and W. L. Deng, Nanoscale, 2015, 7, 11734–11745 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed description of experimental section and additional STM images. See DOI: 10.1039/c5ra18434g |
| This journal is © The Royal Society of Chemistry 2015 |