Preparation of a nanoporous CuO/Cu composite using a dealloy method for high performance lithium-ion batteries
Abstract
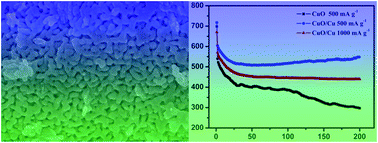
Nanoporous CuO/Cu composite materials with a bicontinuous structure are prepared by corroding a Cu–Al alloy. The obtained nanoporous CuO/Cu composites exhibit excellent performance as anodes for lithium ion batteries, which show a capacity of 600 mA h g−1 at a current density of 500 mA g−1 and maintain the capacity for 200 cycles and 445 mA h g−1 at 1000 mA g−1. The excellent electrochemical performance is attributed to the nanoporous and Cu-doped structure.

 Please wait while we load your content...
Please wait while we load your content...