Rational selection of solvents and fine tuning of morphologies toward highly efficient polymer solar cells fabricated using green solvents†
Delong Liua,
Zaiyu Wangb,
Shaoqing Zhanga,
Zhong Zhenga,
Bei Yanga,
Wei Ma*b and
Jianhui Hou*a
aLaboratory of Polymer Physics and Chemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: hjhzlz@iccas.ac.cn
bState Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, Xi'an 710049, China 710049. E-mail: msewma@mail.xjtu.edu.cn
First published on 10th August 2015
Abstract
High-efficiency polymer solar cells (PSCs) based on a conjugated polymer named PBQ-4 were fabricated by employing 1,2-dichlorobenzene (o-DCB), o-xylene and anisole as primary solvents. We rationally selected three solvent additives, 1,8-diiodooctane (DIO), N-methylpyrrolidone (NMP) and diphenyl ether (DPE) to tune the morphologies of the blend films. The power conversion efficiency (PCE) of 8.47% and 7.62% were realized by using o-DCB/DIO and o-xylene/NMP as processing solvent, respectively. The PSC with a PCE of 8.37% was fabricated using the environmentally friendly solvents of anisole/DPE. To the best of our knowledge, the PCE of 8.37% is not only among the highest values reported for PSCs with Eg > 1.7 eV but also the highest value for a PSC processed using a biodegradable solvents with low toxicity. Therefore, these results open the new paths for the fabrication of highly efficient PSCs by green processes.
1. Introduction
In the past few decades, polymer solar cells (PSCs) with a bulk heterojunction (BHJ) structure have attracted considerable attention because of their easy processability, high flexibility and low weight.1–5 The power conversion efficiency (PCE) of single-junction PSCs has been pushed to over 9%.6–10 The morphology of the BHJ blend, which is composed of a conjugated polymer (donor) and a fullerene derivative (acceptor), plays a critical role in achieving a high PCE.11–14 As is well known, a nanoscale bicontinuous interpenetrating network is beneficial for realizing efficient exciton dissociation and charge transport.15,16 Various methods have been employed to tune the morphology of BHJ-blend films, including donor/acceptor (D/A) ratio control, thermal annealing, solvent annealing and the use of varied primary solvents and solvent additives.17–22 Amongst these strategies, the selection of suitable primary solvents and solvent additives has been widely adopted to optimize the morphology of the active layer.23–26At present, halogenated solvents such as chloroform (CF), chlorobenzene (CB) and 1,2-dichlorobenzene (o-DCB) are predominately used in the fabrication of PSCs because of their ability to solvate conjugated polymers and fullerene derivatives, and the PSCs processed using these solvents have exhibited outstanding device performance. However, the use of these solvents is prohibited in industry because they are detrimental to the environment and to human health. Therefore, the use of environmentally friendly solvents has drawn much attention in the field of PSCs.27,28 For example, Jen et al. used the non-halogenated solvents o-xylene, 1,2,4-trimethylbenzene and 1,2-dimethylnaphthalene to fabricate PSCs with PIDTT-DFBT:PC71BM as the active layer and achieved a PCE of 7.26%.29 Li et al. used toluene as the processing solvent and achieved a PCE of 6.56% in PSCs based on P3HT:ICBA.30 Based on a new polymer PBDTTT-TEG, a device processed with N-methylpyrrolidone (NMP) exhibited a PCE of 5.23%.31 Recently, a PSC device with at PCE of 5.1% was fabricated using 2-methyltetrahydrofuran as the processing solvent.32 Thus far, PSCs fabricated from relatively safe solvents have exhibited inferior photovoltaic performance compared to that of PSCs prepared from halogenated solvents.
Generally, to modify the morphologies of the BHJ layers, the primary solvent and solvent additive must exhibit different solvation ability toward the conjugated polymers and fullerene derivatives, and the boiling point of the solvent additive must be higher than that of the primary solvent.33 Compared to the highly toxic halogenated aromatic solvents, aromatic ethers are promising candidates for the fabrication of efficient PSCs because of their merits of low toxicity, good biodegradability and pleasant odor. Recently, a few research groups have started to employ aromatic ethers as the primary solvent or solvent additive for PSC device fabrication. For instance, Qiao et al. used anisole as the solvent to process PSCs with a PBT-T1/PC61BM active layer and achieved a PCE of 2.52%.34 Woo et al. reported a PCE of 9.39% in PSCs based on the PPDT2FBT:C71BM system and processed with CB as the primary solvent and diphenyl ether (DPE) as the solvent additive.35 Heeger et al. achieved a PCE greater than 9% with a thick film of ca. 340 nm through the addition of DPE as the optimum processing additive to the blend system of DT-PDPP2T-TT:C71BM.10 According to these examples, we conclude that aromatic ethers can be successfully used as a solvent additive, although the photovoltaic performance of PSCs fabricated using aromatic ethers as the primary processing solvent still substantially lags that of PSCs processed using other types of primary solvents.
Recently, we designed and synthesized a fluorine-substituted benzo[1,2-b:4,5-b′]dithiophene (BDT-T-2F) building block and prepared BDT-T-2F-based conjugated polymers with a deep highest occupied molecular orbital (HOMO) level.36 When BDT-T-2F was copolymerized with 6,7-difluoro-5,8-dibromo-2,3-bis(3-(2-ethylhexyloxy)phenyl)-quinoxaline (DTQx-2F), a new polymer named PBQ-4 was prepared (see Scheme 1 and S1 in ESI†). We observed that PBQ-4 was easily dissolved in many different aromatic solvents, including o-DCB, o-xylene, and anisole; hence, we had the opportunity to fabricate PSCs using PBQ-4:PC71BM ([6,6]-phenyl-C71-butyric acid methyl ester) as the photoactive material and three aromatic solvents with different toxicities, i.e., o-DCB, o-xylene and anisole, as the primary processing solvent. Surprisingly, over 8% efficiency was achieved using anisole as the primary solvent and DPE as the solvent additive. Moreover, a variety of measurements, including transmission electron microscopy (TEM), atomic force microscopy (AFM), grazing-incidence wide-angle X-ray scattering (GIWAXS) and resonant soft X-ray scattering (R-SoXS), were employed to investigate the effects of the processing solvents on bulk and surface morphologies of the PBQ-4:PC71BM blend.
2. Results and discussion
Scheme 1 shows the molecular structures of the active layer materials and the solvents used for fabricating the PSCs. A device architecture of ITO/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS)/BHJ blend/Ca/Al was used in this work. First, o-DCB and 1,8-diiodooctane (DIO) were used as the primary solvent and additive to fabricate the control device. We observed that the optimal ratio of PBQ-4:PC71BM (D/A ratio, wt/wt) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
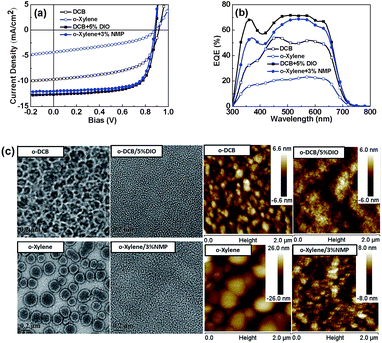
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 and that the optimal amount of DIO additive is 5% (v/v) (see Fig. S1a†). Fig. 1a shows the typical current density vs. voltage (J–V) characteristics of PBQ-4:PC71BM solar cells under AM 1.5G illumination (100 mW cm−2). Under the optimized conditions, the devices prepared using o-DCB/DIO yielded an optimal PCE of 8.47%, with a VOC of 0.89 V, a JSC of 13.68 mA cm−2 and an FF of 69.53%.
1.5 and that the optimal amount of DIO additive is 5% (v/v) (see Fig. S1a†). Fig. 1a shows the typical current density vs. voltage (J–V) characteristics of PBQ-4:PC71BM solar cells under AM 1.5G illumination (100 mW cm−2). Under the optimized conditions, the devices prepared using o-DCB/DIO yielded an optimal PCE of 8.47%, with a VOC of 0.89 V, a JSC of 13.68 mA cm−2 and an FF of 69.53%.
As described in our recent work,37 a mixture of o-xylene and NMP can be used as a universal halogen-free solvent system to replace the highly toxic solvent mixture of o-DCB and DIO. Herein, we fabricated the PSCs based on PBQ-4:PC71BM by using o-xylene as the primary solvent and NMP as an additive. When the blend film of PBQ-4:PC71BM was spin-coated using pure o-xylene as the processing solvent, the device exhibited a PCE of 1.83%; when the blend film was spin-coated using in o-xylene containing 3% NMP (v/v) as the processing solvent, the JSC was enhanced from 4.24 mA cm−2 to 12.01 mA cm−2 and the FF was improved from 44% to 72%. As a result, the use of o-xylene/NMP as the processing solvent led to a PCE of 7.62%.
On the basis of the detailed photovoltaic results listed in Table 1, we concluded that the photovoltaic performance of the devices processed using o-DCB or o-xylene as the primary solvent can be significantly improved by adding a trace amount of high-boiling-point additive. To determine the influence of the additives on the morphologies of the blends of PBQ-4:PC71BM, the bulk and surface morphologies of the blends were investigated by TEM and AFM measurements. As shown in Fig. 1c, when the PBQ-4:PC71BM films were cast using o-DCB without an additive, large-scale aggregates of PC71BM (100–200 nm) were observed, which may cause severe germinate recombination;38 in the case of the blend film processed by o-DCB/DIO, the large-size aggregates of PC71BM were diminished and the blend film became very uniform. A similar phenomenon was observed for the blend film processed using pure o-xylene or o-xylene/NMP; we therefore reasonably conclude that when o-DCB or o-xylene is used as the primary solvent, the addition of DIO or NMP can reduce the phase separation in the blend of PBQ-4:PC71BM and lead to a significant improvement of photovoltaic performance.
| Processing solvent | VOC (V) | JSC (mA cm−2) | JSCa (mA cm−2) | FF (%) | PCE (%) | Thick-nessc (nm) | |
|---|---|---|---|---|---|---|---|
| Avg.b | Best | ||||||
| a Calculated from the EQE spectra.b Average data obtained from six devices.c The thickness of active layer in optimal devices. | |||||||
| o-DCB | 0.92 | 9.67 | 9.26 | 58.2 | 5.10 | 5.24 | 87 |
| o-Xylene | 0.90 | 4.24 | 4.03 | 44.0 | 1.74 | 1.83 | 94 |
| Anisole | 0.90 | 7.80 | 7.26 | 51.1 | 3.51 | 3.60 | 90 |
| o-DCB + 5% DIO | 0.89 | 13.68 | 12.72 | 69.5 | 8.32 | 8.47 | 83 |
| o-Xylene + 3% NMP | 0.88 | 12.01 | 11.73 | 72.0 | 7.50 | 7.62 | 95 |
| Anisole + 3% DPE | 0.88 | 12.64 | 12.26 | 75.2 | 8.28 | 8.37 | 92 |
| Anisole + 1% DIO | 0.87 | 12.60 | 12.53 | 75.4 | 8.22 | 8.28 | 90 |
| Anisole + 1% NMP | 0.87 | 11.79 | 11.71 | 71.8 | 7.16 | 7.34 | 90 |
As previously mentioned, the polymer PBQ-4 can be easily dissolved into anisole. We observed that PC71BM exhibits good solubility in anisole (65 mg mL−1), similar to its solubility in o-xylene (see Table 2). Therefore, we used anisole as the processing solvent to fabricate PSCs based on PBQ-4:PC71BM. As shown in Fig. 2a, when pure anisole was used, a PCE of 3.60% was recorded, with a JSC of 7.80 mA cm−2, a VOC of 0.90 V and an FF of 51.1%. As shown in the TEM and AFM topography images shown in Fig. 3, large-sized aggregates are clearly observed in the PBQ-4:PC71BM blend processed using pure anisole, similar to what was observed in the blend film processed using pure o-DCB or o-xylene. With respect to the morphologies of the blend films processed using o-DCB or o-xylene as the primary solvent, we predicted that the photovoltaic performance of the device processed by anisole could be significantly improved through the use of an appropriate solvent additive.
| Solvent | Solubility of PBQ-4 (mg mL−1) | Solubility of PC71BM (mg mL−1) | Boiling pointa (°C) | Toxicity/LD50a (mg kg−1) | Environmental issues |
|---|---|---|---|---|---|
| a Data obtained from Materials Safety Data Sheet (MSDS) files provided by Sigma-Aldrich (corrected to 760 mm Hg).b No data available in the MSDS files from Sigma-Aldrich and other databases.c Data obtained from ref. 24.d Data obtained from ref. 31. | |||||
| o-DCB | >50 | 203c | 180.0 | 500 | Hazardous & accumulative |
| DIO | Insoluble | 63d | 326 | N/Ab | N/Ab |
| o-Xylene | >50 | 66.2c | 144.5 | 1364 | Low risk (biodegradable) |
| NMP | Insoluble | 48d | 202 | 3598 | Low risk (biodegradable) |
| Anisole | >15 | 65 | 153.7 | 3700 | Low risk (biodegradable) |
| DPE | <1 | 236 | 258.0 | 3370 | Low risk |
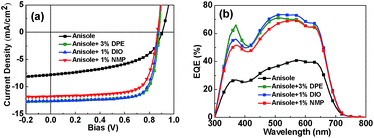 | ||
| Fig. 2 (a) J–V curves of PSCs based on PBQ-4/PC71BM processed with pure anisole, anisole/3% DPE, anisole/1% DIO and anisole/1% NMP. (b) EQE curves of the corresponding devices. | ||
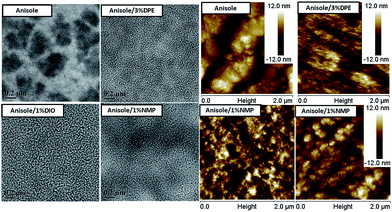 | ||
| Fig. 3 TEM and topography images of PBQ-4:PC71BM films processed with anisole, anisole/3% DPE, anisole/1% DIO, and anisole/1% NMP. | ||
To enable the rational selection of solvent additives, various basic properties of the solvents used in this work were considered; these properties are summarized in Table 2. Both PBQ-4 and PC71BM clearly exhibit good solubility in o-DCB and o-xylene, whereas DIO and NMP are poor solvents for PBQ-4 but relatively good solvents for PC71BM. In comparison with DIO and NMP, o-DCB and o-xylene possess much lower boiling points. Therefore, when anisole is used as the primary solvent, a high-boiling-point solvent with poor solvating ability toward PBQ-4 but good solvating ability toward PC71BM may be a suitable solvent additive; thus, DIO and NMP were used as additives. Furthermore, when we investigated the properties of DPE, we observed that this solvent exhibits some unique properties and is a potential additive for use in combination with anisole. For example, the boiling point of DPE (258 °C) is higher than that of anisole (153.7 °C); DPE is a poor solvent for PBQ-4 but an excellent solvent for PC71BM; and, unlike NMP and DIO, DPE is a biodegradable compound with low toxicity and a pleasant odor and can even be used as an additive in food. Therefore, to tune the morphologies of the PBQ-4:PC71BM blend processed using anisole, we used DPE, DIO and NMP as the solvent additives.
Initially, the optimal additive concentrations of DPE, DIO and NMP were determined by varying the feed ratios of the additives. As shown in Fig. S1 and Table S1,† when anisole is used as the primary solvent, the optimal ratios for DPE, DIO and NMP are 3%, 1% and 1%, respectively. The J–V curves of the devices fabricated under the optimal conditions are shown in Fig. 2a, and the corresponding photovoltaic data are listed in Table 1. The device processed using anisole and 3% DPE exhibited a PCE of 8.37%, which is almost the same as that of the device processed using o-DCB and 5% DIO. In the case of DIO and NMP as additives, the optimized devices exhibited PCEs of 8.28% and 7.34%, respectively.
On the basis of the photovoltaic results obtained for the devices and the intrinsic properties of the solvents used in this work, we roughly evaluated the practical potential of the results. In recent years, the international community has instituted tight controls on persistent organic pollutants (POPs). POPs are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes.39 Therefore, the ideal solvents for the fabrication of PSCs must exhibit low toxicity and be biodegradable, and, equally important, the PSC devices prepared using such solvents must be highly efficient. Because a thorough evaluation of the toxicity of a chemical is difficult, we used only the lethal median dose (LD50), as shown in Table 2, to evaluate the toxicity of the solvents. The LD50 of both anisole and DPE exceed 3000 mg kg−1, which is much higher than that of o-DCB and o-xylene, indicating that anisole and DPE are safer than o-DCB and o-xylene. o-DCB can cause accumulative pollution; in contrast, both anisole and DPE are biodegradable chemicals and can be used as additives in food. Furthermore, the PSCs fabricated using anisole and DPE exhibited a PCE of 8.37%, which is almost the same as that of the devices fabricated using o-DCB and DIO. Therefore, with respect to the fabrication of a PBQ-4:PC71BM-based PSC with high photovoltaic performance using a green process, the mixture of anisole and DPE is an ideal processing solvent.
The external quantum efficiency (EQE) curves of the devices are shown in Fig. 2b. For the device processed using pure anisole, the EQE curve exhibits a peak of ca. 40%, whereas the EQE peaks of the devices processed using solvents with additives reach over 70%; as a result, the integral current densities obtained from the EQE measurements can be improved from 7.8 mA cm−2 to over 12 mA cm−2. To determine the cause for the enhancement in current density, we investigated the morphologies of the corresponding PBQ-4:PC71BM films by TEM and AFM. As shown in Fig. 3, in comparison with the blend film processed using pure anisole, the sizes of aggregates in the blend films processed using anisole and an additive were effectively reduced. As is known, the exciton diffusion length in organic semiconductors is limited to within ∼10 nm because of their low dielectric constant, and smaller aggregates in the BHJ blend favor a reduction of geminate recombination in PSC devices; thus, the use of an additive enhances the JSC of the resulting device.
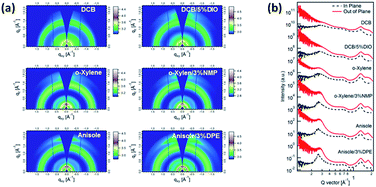
To provide valuable insights into the molecular-scale packing of the PBQ-4:PC71BM blend films processed using different solvents, GIWAXS was performed to probe the crystalline regions. 2D GIWAXS patterns and out-of-plane (OOP) and in-plane (IP) reflection profiles of the PBQ-4:PC71BM blend cast from different solvent mixtures are shown in Fig. 4. For the film processed with pure o-DCB, a weak OOP diffraction peak appeared at q = 1.70 Å, corresponding to the interchain π–π stacking. The materials in these blend films are concluded to preferentially exhibit an on-orientation structure with respect to the substrate.40 The diffraction halo at q = 1.40 Å is typically attributed to the amorphous scattering from the PC71BM within the blend. When the film was processed with DCB/5% DIO, the (010) diffraction peak became slightly sharper and its position shifted to a lower q (= 1.68 Å), corresponding to an increase in π–π stacking. We attribute these changes to a more ordered crystalline array of PBQ-4 resulting from the use of the additive DIO, which was also confirmed by the coherence length (CL) (calculated using the Scherrer equation and reported in Table S2†).41 Similar results were observed in the cases of o-xylene and anisole with or without additives. Moreover, the CL corresponding to the films processed with DCB/5% DIO, o-xylene/3% NMP and anisole/3% DPE was gradually enhanced. The CL of π–π stacking is intimately correlated with the charge transport in the blend. To investigate the charge transport properties of blend films processed using different solvent systems, hole mobilities were measured using the space-charge-limited current (SCLC) method with hole- and electron-only devices.42 The hole mobilities of blend films processed using o-DCB/DIO, o-xylene/NMP and anisole/DPE were 1.29 × 10−4 cm2 V−1 s−1, 2.19 × 10−4 cm2 V−1 s−1 and 2.87 × 10−4 cm2 V−1 s−1, respectively. The CL trend of these three blend films is consistent with the hole mobility and FF because the improved π–π stacking ordering is favorable for charge transport, thus leading to the enhanced hole mobility; higher hole mobility, in turn, reduces bimolecular recombination and results in an improved FF.
Because the phase separation of domains is critical to exciton diffusion, dissociation and charge transport, R-SoXS was used to provide detailed information about the bulk nanostructure of BHJ films processed using optimized solvent mixtures.43–45 Fig. 5 shows the scattering profiles for the films spin-coated from o-DCB/5% DIO, o-xylene/3% NMP and anisole/3% DPE. The film processed using DCB/5% DIO exhibits the smallest median domain size of ∼10 nm. The relative small domain size was identified as being necessary for the effective exciton dissociation (due to the exciton diffusion length of approximately 10 nm), which enhances the JSC.46–48 This observation is consistent with the JSC of the device processed using o-DCB/5% DIO exhibiting the highest JSC of 13.68 mA cm−2. However, the films processed using o-xylene/3% NMP and anisole/3% DPE contain broad distributions of domain sizes. The median domain sizes are ∼30–40 nm for the blends processed using o-xylene/3% NMP and anisole/3% DPE. This domain size is slightly larger than that of blends processed using o-DCB/5% DIO; thus, a slightly smaller JSC was obtained. The relative domain purity can be verified by calculating the total scattering intensity (TSI).49 The relative domain purity is 50%, 97%, and 100% for blends processed using o-DCB/5% DIO, o-xylene/3% NMP and anisole/3% DPE, respectively. Pure domains have been reported to decrease the detrimental bimolecular charge recombination, which can reasonably account for the FF as high as 75% obtained from the film processed using anisole/3% DPE.46,50,51 The relative domain purity is also consistent with π–π stacking and hole mobility, as previously discussed.
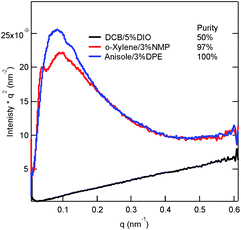 | ||
| Fig. 5 Resonant soft X-ray scattering (R-SoXS) profiles (284.8 eV) of blend films processed using optimal solvent mixtures. | ||
3. Conclusions
In conclusion, we synthesized a conjugated polymer, PBQ-4, and fabricated efficient PSCs based on this polymer by employing a variety of solvents. According to the photovoltaic and morphological properties of the PSCs processed using four types of processing solvents, including o-DCB, o-DCB/DIO, o-xylene and o-xylene/NMP, we rationally selected three solvent additives to tune the morphologies of the PBQ-4:PC71BM blend films processed using anisole. The photovoltaic results indicated that these three additives (i.e., DPE, DIO, and NMP) work well for preventing severe phase separation in the PBQ-4:PC71BM blends. We also investigated the morphological properties of the blend films through various methods and correlated the photovoltaic properties of the PBQ-4:PC71BM blends with their morphologies and with the nature of the processing solvents. Therefore, the results and conclusions in this work can be used as a guideline for the rational selection of processing solvents in PSCs. More importantly, the PSCs fabricated using anisole/DPE exhibited a PCE of 8.37%, which is higher than that of the devices processed using o-xylene/NMP and almost the same as that of the devices fabricated using o-DCB/DIO. Because both anisole and DPE are biodegradable chemicals with very low toxicity, the mixture of anisole and DPE can be considered a green processing solvent for fabricating PBQ-4:PC71BM-based PSCs. To the best of our knowledge, the PCE of 8.37% is not only among the highest values reported for PSCs with Eg > 1.7 eV but is also the highest value for a PSC processed using a biodegradable solvents with low toxicity. Therefore, we expect this work to open new paths for the fabrication of highly efficient PSCs by green processes.Acknowledgements
The authors would like to acknowledge the financial support from National Basic Research Program 973 (2014CB643501), Ministry of Science and Technology of China, NSFC (No. 91333204, 21325419, 51173189), the Chinese Academy of Science (No. XDB12030200). X-ray data was acquired at beamlines 7.3.3 and 11.0.1.2 at the Advanced Light Source, which is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.Notes and references
- J. G. G. Yu, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 Search PubMed.
- S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef PubMed.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153 CrossRef CAS PubMed.
- Y. Li, Acc. Chem. Res., 2012, 45, 723 CrossRef CAS PubMed.
- X. G. Guo, N. J. Zhou, S. J. Lou, J. Smith, D. B. Tice, J. W. Hennek, R. P. Ortiz, J. T. L. Navarrete, S. Y. Li, J. Strzalka, L. X. Chen, R. P. H. Chang, A. Facchetti and T. J. Marks, Nat. Photonics, 2013, 7, 825 CrossRef CAS PubMed.
- Y. Yang, W. Chen, L. Dou, W.-H. Chang, H.-S. Duan, B. Bob, G. Li and Y. Yang, Nat. Photonics, 2015, 9, 190 CrossRef CAS PubMed.
- Z. He, B. Xiao, F. Liu, H. Wu, Y. Yang, S. Xiao, C. Wang, T. P. Russell and Y. Cao, Nat. Photonics, 2015, 9, 174 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- S. Zhang, L. Ye, W. Zhao, B. Yang, Q. Wang and J. Hou, Sci. China: Chem., 2015, 58, 248 CrossRef CAS.
- H. Choi, S. J. Ko, T. Kim, P. O. Morin, B. Walker, B. H. Lee, M. Leclerc, J. Y. Kim and A. J. Heeger, Adv. Mater., 2015, 27, 3318 CrossRef CAS PubMed.
- M. T. Dang, L. Hirsch, G. Wantz and J. D. Wuest, Chem. Rev., 2013, 113, 3734 CrossRef CAS PubMed.
- S. Kouijzer, J. J. Michels, M. van den Berg, V. S. Gevaerts, M. Turbiez, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2013, 135, 12057 CrossRef CAS PubMed.
- Y. Huang, E. J. Kramer, A. J. Heeger and G. C. Bazan, Chem. Rev., 2014, 114, 7006 CrossRef CAS PubMed.
- M. Campoy-Quiles, T. Ferenczi, T. Agostinelli, P. G. Etchegoin, Y. Kim, T. D. Anthopoulos, P. N. Stavrinou, D. D. C. Bradley and J. Nelson, Nat. Mater., 2008, 7, 158 CrossRef CAS PubMed.
- L. Ye, S. Zhang, W. Ma, B. Fan, X. Guo, Y. Huang, H. Ade and J. Hou, Adv. Mater., 2012, 24, 6335 CrossRef CAS PubMed.
- W. Ma, J. R. Tumbleston, L. Ye, C. Wang, J. Hou and H. Ade, Adv. Mater., 2014, 26, 4234 CrossRef CAS PubMed.
- X. Guo, M. J. Zhang, J. H. Tan, S. Q. Zhang, L. J. Huo, W. P. Hu, Y. F. Li and J. H. Hou, Adv. Mater., 2012, 24, 6536 CrossRef CAS PubMed.
- G. Li, V. Shrotriya, J. S. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CrossRef CAS PubMed.
- J. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger and G. C. Bazan, Nat. Mater., 2007, 6, 497 CrossRef CAS PubMed.
- F. S. U. Fischer, D. Trefz, J. Back, N. Kayunkid, B. Tornow, S. Albrecht, K. G. Yager, G. Singh, A. Karim, D. Neher, M. Brinkmann and S. Ludwigs, Adv. Mater., 2015, 27, 1223 CrossRef CAS PubMed.
- G. Li, Y. Yao, H. Yang, V. Shrotriya, G. Yang and Y. Yang, Adv. Funct. Mater., 2007, 17, 1636 CrossRef CAS PubMed.
- Y. Gu, C. Wang and T. P. Russell, Adv. Energy Mater., 2012, 2, 683 CrossRef CAS PubMed.
- W. L. Ma, C. Y. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617 CrossRef CAS PubMed.
- B. Walker, A. Tamayo, D. T. Duong, X.-D. Dang, C. Kim, J. Granstrom and T.-Q. Nguyen, Adv. Energy Mater., 2011, 1, 221 CrossRef CAS PubMed.
- K. R. Graham, P. M. Wieruszewski, R. Stalder, M. J. Hartel, J. Mei, F. So and J. R. Reynolds, Adv. Funct. Mater., 2012, 22, 4801 CrossRef CAS PubMed.
- J. K. Lee, W. L. Ma, C. J. Brabec, J. Yuen, J. S. Moon, J. Y. Kim, K. Lee, G. C. Bazan and A. J. Heeger, J. Am. Chem. Soc., 2008, 130, 3619 CrossRef CAS PubMed.
- P.-T. Tsai, C.-Y. Tsai, C.-M. Wang, Y.-F. Chang, H.-F. Meng, Z.-K. Chen, H.-W. Lin, H.-W. Zan, S.-F. Horng, Y.-C. Lai and P. Yu, Org. Electron., 2014, 15, 893 CrossRef CAS PubMed.
- J.-H. Chang, H.-F. Wang, W.-C. Lin, K.-M. Chiang, K.-C. Chen, W.-C. Huang, Z.-Y. Huang, H.-F. Meng, R.-M. Ho and H.-W. Lin, J. Mater. Chem. A, 2014, 2, 13398 CAS.
- C.-C. Chueh, K. Yao, H.-L. Yip, C.-Y. Chang, Y.-X. Xu, K.-S. Chen, C.-Z. Li, P. Liu, F. Huang, Y. Chen, W.-C. Chen and A. K. Y. Jen, Energy Environ. Sci., 2013, 6, 3241 CAS.
- X. Guo, M. Zhang, C. Cui, J. Hou and Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 8190 CAS.
- Y. Chen, S. Q. Zhang, Y. Wu and J. H. Hou, Adv. Mater., 2014, 26, 2744 CrossRef CAS PubMed.
- X. Chen, X. Liu, M. A. Burgers, Y. Huang and G. C. Bazan, Angew. Chem., Int. Ed., 2014, 53, 14378 CrossRef CAS PubMed.
- J. Peet, N. S. Cho, S. K. Lee and G. C. Bazan, Macromolecules, 2008, 41, 8655 CrossRef CAS.
- S. Venkatesan, Q. L. Chen, E. C. Ngo, N. Adhikari, K. Nelson, A. Dubey, J. Y. Sun, V. Bommisetty, C. Zhang, D. Galipeau and Q. Q. Qiao, Energy Technol., 2014, 2, 269 CrossRef CAS PubMed.
- T. L. Nguyen, H. Choi, S. J. Ko, M. A. Uddin, B. Walker, S. Yum, J. E. Jeong, M. H. Yun, T. J. Shin, S. Hwang, J. Y. Kim and H. Y. Woo, Energy Environ. Sci., 2014, 7, 3040 CAS.
- M. J. Zhang, X. Guo, S. Q. Zhang and J. H. Hou, Adv. Mater., 2014, 26, 1118 CrossRef CAS PubMed.
- W. Zhao, L. Ye, S. Zhang, M. Sun and J. Hou, J. Mater. Chem. A, 2015, 3, 12723 CAS.
- A. Maurano, R. Hamilton, C. G. Shuttle, A. M. Ballantyne, J. Nelson, B. O'Regan, W. Zhang, I. McCulloch, H. Azimi, M. Morana, C. J. Brabec and J. R. Durrant, Adv. Mater., 2010, 22, 4987 CrossRef CAS PubMed.
- L. Ritter, K. R. Solomon, J. Forget, M. Stemeroff and C. O'Leary, Persistent organic pollutants, United Nations Environment Programme, 2007 Search PubMed.
- I. Osaka, M. Saito, T. Koganezawa and K. Takimiya, Adv. Mater., 2014, 26, 331 CrossRef CAS PubMed.
- J. T. Rogers, K. Schmidt, M. F. Toney, E. J. Kramer and G. C. Bazan, Adv. Mater., 2011, 23, 2284 CrossRef CAS PubMed.
- G. G. Malliaras, J. R. Salem, P. J. Brock and C. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, R13411 CrossRef CAS.
- M. J. Zhang, X. Guo, W. Ma, S. Q. Zhang, L. J. Huo, H. Ade and J. H. Hou, Adv. Mater., 2014, 26, 2089 CrossRef CAS PubMed.
- D. Chen, F. Liu, C. Wang, A. Nakahara and T. P. Russell, Nano Lett., 2011, 11, 2071 CrossRef CAS PubMed.
- H. Yan, B. A. Collins, E. Gann, C. Wang, H. Ade and C. R. McNeill, ACS Nano, 2012, 6, 677 CrossRef CAS PubMed.
- W. Ma, J. R. Tumbleston, M. Wang, E. Gann, F. Huang and H. Ade, Adv. Energy Mater., 2013, 3, 864 CrossRef CAS PubMed.
- R. R. Lunt, N. C. Giebink, A. A. Belak, J. B. Benziger and S. R. Forrest, J. Appl. Phys., 2009, 105, 053711 CrossRef PubMed.
- P. E. Shaw, A. Ruseckas and I. D. W. Samuel, Adv. Mater., 2008, 20, 3516 CrossRef CAS PubMed.
- B. A. Collins, Z. Li, J. R. Tumbleston, E. Gann, C. R. McNeill and H. Ade, Adv. Energy Mater., 2013, 3, 65 CrossRef CAS PubMed.
- A. M. Ballantyne, T. A. M. Ferenczi, M. Campoy-Quiles, T. M. Clarke, A. Maurano, K. H. Wong, W. Zhang, N. Stingelin-Stutzmann, J.-S. Kim, D. D. C. Bradley, J. R. Durrant, I. McCulloch, M. Heeney, J. Nelson, S. Tierney, W. Duffy, C. Mueller and P. Smith, Macromolecules, 2010, 43, 1169 CrossRef CAS.
- S. Albrecht, S. Janietz, W. Schindler, J. Frisch, J. Kurpiers, J. Kniepert, S. Inal, P. Pingel, K. Fostiropoulos, N. Koch and D. Neher, J. Am. Chem. Soc., 2012, 134, 14932 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details for monomers, additional GIWAXS and photovoltaic characterization data. See DOI: 10.1039/c5ra14013g |
| This journal is © The Royal Society of Chemistry 2015 |