Structure–property studies of P-triarylamine-substituted dithieno[3,2-b:2′,3′-d]phospholes†
Hannes Puntscher‡
ab,
Paul Kautny‡a,
Berthold Stögerc,
Antoine Tissotd,
Christian Hametnera,
Hans R. Hagemannd,
Johannes Fröhlicha,
Thomas Baumgartner*b and
Daniel Lumpi*a
aInstitute of Applied Synthetic Chemistry, Vienna University of Technology, Getreidemarkt 9/163, A-1060 Vienna, Austria. E-mail: daniel.lumpi@tuwien.ac.at
bDepartment of Chemistry & Centre for Advanced Solar Materials, University of Calgary, 2500 University Dr. NW, Calgary, AB T2N 1N4, Canada. E-mail: ttbaumga@ucalgary.ca
cInstitute of Chemical Technologies and Analytics, Vienna University of Technology, Getreidemarkt 9/164, A-1060 Vienna, Austria
dDépartement de Chimie Physique, Université de Genève, 30, quai E. Ansermet, 1211 Geneva 4, Switzerland
First published on 26th October 2015
Abstract
The synthesis of 10 novel P-substituted dithienophosphole oxide compounds applying phenylcarbazole and indolocarbazole donors is presented. Based on photo-physical and theoretical investigations, the study reveals that the pyramidal geometry of the phosphorus allows for the synthesis of charge transfer materials by introducing strong exocyclic donor groups but suppresses intramolecular charge transfer below a certain donor strength threshold, which is an appealing structural feature for the design of donor–acceptor materials. The triplet energies of the phenylcarbazole based compounds are in the range of 2.49–2.65 eV, sufficiently high for potential applications as host materials in PhOLEDs. By contrast, the introduction of indolocarbazole, the weakest employed donor, yields materials exhibiting a significantly higher triplet energy of up to 2.87 eV and a remarkably low singlet–triplet splitting (0.18 eV). In addition an interesting example of an intramolecular electronic through-space interaction has been observed for the ortho-linked phenylcarbazole derivative.
1 Introduction
The development of functional π-conjugated organic materials for applications in organic electronics such as organic light emitting diodes (OLEDs),1–3 organic field effect transistors (OFETs),4–6 organic photovoltaics (OPVs)7,8 or sensors5,6 is a steadily evolving field of research due to the appealing possibility of tailoring the organic materials' intrinsic electronic properties by subtle modifications of their molecular framework.9 The incorporation of main group elements such as B,10–12 Si,13–15 Se,16 Te17 or P18–22 has proven to be a particularly valuable tool for designing materials featuring, e.g., highly interesting optical properties, inaccessible through purely hydrocarbon-based compounds.In this context, the dithieno[3,2-b:2′,3′-d]phosphole scaffold (Fig. 1) has been thoroughly investigated as novel platform for functional materials in the last decade.23 The electron lone pair of the phosphorus atom within the phosphole structure has high s-character that hinders efficient interaction with the π-system.23 Therefore, the aromaticity of phospholes is diminished compared to other five-membered heterocycles, such as pyrrole, thiophene or furan. Nevertheless, interactions between the σ*-orbital of the exocyclic bond and the π*-system of the ring lead to a certain degree of aromaticity and a high polarizability of the phosphole system.23,24 The resulting high tunability of the photo-physical and electro-chemical properties by chemical modification22,23,25 suggested the utility of this versatile building block for the fields of luminescent materials,26–31 polymers,32–34 coordination chemistry26,35 and self-organizing materials.36,37 In particular, oxidation of the phosphorus center is an appealing strategy to significantly enhance the electron-accepting properties of the dithieno[3,2-b:2′,3′-d]phosphole moiety,21,24 which enables the application of this scaffold in donor–acceptor type materials.
Donor–acceptor materials are of specific interest for applications due to the possibility to selectively influence photo-physical and electro-chemical characteristics by intramolecular charge transfer (ICT)2,3,38–40 as well as bipolar charge transport properties in electronic devices.39,41 Thus, the interaction of the molecular donor and acceptor subunits is of crucial importance. Phosphine oxide derivatives have been widely applied as functional materials in OLEDs.21 Particularly bipolar host materials for phosphorescent OLEDs (PhOLEDs) comprising phosphine oxide and carbazole42–44 or triphenylamine45,46 moieties were exhaustively investigated due to limited conjugation via the phosphine oxide as result of its tetrahedral geometry.21 Moreover, the coordination geometry of the phosphorus atom in the five-membered phosphole ring offers new opportunities to control electronic and photo-physical characteristics.22–24 Whereas direct substitution of the thiophene moieties allows for full conjugation with the main scaffold, the pyramidal structure of the PC3 fragment prevents π-conjugation of the exocyclic substituent with the dithieno[3,2-b:2′,3′-d]phosphole core.
Recently, the influence of exocyclic donor groups on the properties of dithieno[3,2-b:2′,3′-d]phosphole based donor–acceptor materials has been investigated29–31 revealing efficient charge transfer from the donor to the dithieno[3,2-b:2′,3′-d]phosphole oxide core.29 Herein we report on the synthesis and characterization of a new series of dithieno[3,2-b:2′,3′-d]phosphole oxides with phenylcarbazole or indolo[3,2,1-jk]carbazole substituents as exocyclic donor moieties; the experimental results have been correlated with DFT calculations. In order to further elucidate the influence of the donor–acceptor interaction on the photo-physical properties of the whole system, the influence of various substitution patterns, as well as planarization of the donor was investigated.
2 Results and discussion
2.1 Synthesis and characterization
Intrigued by the initial studies on systems with an exocyclic triphenyl amine substituent,29 this work focuses on less electron-donating aryl amines such as phenylcarbazole (PCz; with ortho-, meta- and para-substitution pattern) and indolo[3,2,1-jk]carbazole (ICz). Due to the increasing contribution of the nitrogen lone pair to the π-system of the pyrrole-like rings in the planarized triarylamine (TAA) subunits, the donor strength is reduced in the order triphenylamine > phenylcarbazole > indolocarbazole.47 To circumvent possible insolubility issues as a result of the incorporation of increasingly planarized structural motives, the n-butyl substituted species 3ai–ei were synthesized in addition to target molecules 3a–e without alkyl substituents (Scheme 1). Furthermore, in the case of the meta-linked phenylcarbazole, t-butyl substituents were introduced at the 3- and 6-position of the carbazole unit in order to investigate the influence of these substituents on molecular properties, but to also enhance the electro-chemical stability of the latter.48 Following established procedures, the synthesis of the dithienophospholes was accomplished by lithiation of the corresponding dibromobithiophenes 2 or 2i, followed by conversion of the lithiated species with TAAPCl2 1a–e at low temperature (Scheme 1). The TAAPCl2 starting materials were prepared from the corresponding bromides (TAABr) via suitably adapted literature procedures29 and were applied without further purification. For a streamlined synthetic process, the resulting trivalent phosphorus compounds were not isolated but directly oxidized by addition of excess H2O2 yielding target compounds 3a–3ei in moderate to low yields after column chromatography. | ||
| Scheme 1 Synthetic pathways towards carbazole and indolo[3,2,1-jk]carbazole functionalized dithienophosphole oxides 3a–3ei. | ||
The carbazole-based compounds 3a, 3b and 3d exhibited 31P NMR chemical shifts at δ = 16.2, 17.8 and 18.0 ppm, respectively, that are shifted somewhat upfield compared to the corresponding triphenyl amine-substituted dithienophosphole oxide (cf.: 19.1 ppm).29 In contrast, indolocarbazole-substituted 3e showed a slightly downfield-shifted 31P NMR resonance at δ = 22.2 ppm that is, however, in line with related systems exhibiting exocyclic polyaromatic hydrocarbon (PAH) substituents.30 By contrast, ortho-derivative 3c featured a significantly upfield-shifted 31P NMR chemical shift at δ = 12.9 ppm compared to its congeners 3a and 3b, indicating a distinctively different chemical environment of the phosphorus atom for this particular configuration. In all cases, the n-butyl substituents at the dithienophosphole core led to slightly (1–3 ppm) downfield-shifted resonances, which is in accordance with previous findings.29 In addition, the successful formation of target compounds 3a–3ei was also confirmed by 1H and 13C NMR spectroscopy, as well as high-resolution mass spectroscopy (HRMS).
Moreover, single crystals of 3c (ref. 49) suitable for X-ray crystallography were obtained from a CD2Cl2 solution upon slow evaporation of the solvent at room temperature (Fig. 2). Bond lengths and angles within the dithienophosphole oxide scaffold are in good accordance with previously reported P-phenyl substituted derivatives.29,30 Elongated double bonds and shortened single bonds indicate a high degree of conjugation of the planar scaffold (largest distance of 0.0457(10) Å from the least squares (L.S.) plane observed for the C6 atom). Notably, the carbazole unit deviates distinctly from planarity (distance of the C17 atom from the L.S. plane of 0.1211(10) Å). The most striking feature is the alignment of the dithienophosphole and the carbazole units (Fig. 2 (left)). Due to the ortho-linkage on the phenylene linker, the carbazole is forced in close vicinity to the dithienophosphole unit resulting in a parallel orientation (angle of L.S. planes of 8.26(2)°) of the two subunits. The shortest interatomic contact between both units is only 3.2424(12) Å (C4–C26) hinting toward strong intramolecular π–π interactions. In contrast, no such intermolecular interactions are observed with neighboring molecules (Fig. 2 (right)).
The particular spatial arrangement of the ortho-linked derivatives 3c and 3ci was verified in solution via NMR spectroscopy. Following a complete signal assignment (ESI†) using standard 2D methods, 1D and 2D NOESY spectra were recorded (ESI†). Significant NOE enhancement of the carbazole ortho-proton upon irradiation of both of the thiophene signals (and vice versa) clearly indicates close vicinity of the respective moieties in compound 3c. A similar result was obtained for the same carbazole-H and the remaining thiophene proton for 3ci. Thus, parallel alignment of carbazole and dithienophosphole ring systems in the ortho-bridged molecules 3c and 3ci can be assumed in solution as well.
2.2 Photo-physical investigations
In order to investigate the photo-physical properties of the newly developed materials, UV/Vis absorption and photo-luminescence spectra were recorded in dilute CH2Cl2 solutions; results are summarized in Table 1. The absorption spectra (Fig. 3 (top), ESI†) exhibit specific features of both chromophores – the dithienophosphole and carbazole moieties. Whereas the low wavelength region is dominated by well-resolved transitions at approximately 292, 326 and 338 nm, typical for phenylcarbazoles,50 broad absorption bands, which can be attributed to the dithienophosphole unit,27,35,51 are observed at higher wavelengths (350–400 nm). Notably, peaks arising from the carbazole donors are insensitive to the presence/absence of the n-butyl groups at the dithienophosphole. In contrast, installation of the t-butyl groups at the 3- and 6-position leads to red-shifted carbazole absorption peaks for 3d and 3di. Related transitions are observed for the indolocarbazole-based materials 3e and 3ei. The absorption bands arising from the indolocarbazole unit are slightly blue-shifted compared to those of carbazole, which is in accordance with previously reported results.52 Similarly, the absorption of the dithienophosphole moieties are not influenced by the triarylamines, with exception of 3c and 3ci, but the n-butyl substituents lead to a red-shift of the broad dithienophosphole peak. The optical bandgaps determined from the absorption onset of 3a/b/d/e are located in a narrow range between 3.04 and 3.06 eV. The absorption onsets of n-butyl-substituted 3ai/3bi/3di/3ei are shifted to energies between 2.85 and 2.87 eV likely due to the “+I” effect of the donating butyl substituent (vide infra). However, most striking is the observation that absorption onsets of ortho-derivatives 3c and 3ci are red-shifted by nearly 0.1 eV compared to their respective congeners. The same tendencies are found in emission spectra (Fig. 3 (bottom), ESI†). Whereas 3a/b/d/e exhibit featureless emission maxima around 454 nm, the emission of the n-butyl substituted materials 3ai/bi/di/ei is red-shifted by approximately 30 nm, due to the donor-effect if the alkyl groups (vide infra), while the emission maxima of 3c and 3ci are located at 471.5 and 495.5 nm, respectively. Remarkably, the emission of 3a/b/d/e is identical to the purely phenyl-substituted dithienophosphole oxide (453 nm).32 Therefore, the addition of electron-rich aryl amines does not seem to influence the emission properties of the materials. This is also supported by the DFT calculations that identify the dithienophosphole π*-system as the LUMO for all species with largely comparable HOMO–LUMO energy gaps (vide infra). From these findings – as well as the fact that absorption and emission properties are independent of the kind of the adjacent subunit and linkage mode (with the exception of ortho-linkage) – we conclude that the electronic coupling of both chromophores is basically suppressed by linkage via the phosphorus atom. This finding is in disagreement with previous findings concerning triphenyl amine substituted dithienophosphole oxides that exhibit pronounced ICT,29 but can be attributed to the fact that the donor-strength of the aryl amines applied in this study is significantly decreased compared to triphenyl amine.52 Therefore, the pyramidal nature of the phosphorus allows for the synthesis of charge transfer materials based on strong exocyclic donor groups but suppresses ICT below a certain donor strength threshold. This appealing structural feature for the design of donor–acceptor materials has also been confirmed via the DFT calculations (vide infra).| Compound | λabs [nm] | λem [nm] | ESa [eV] | ETb [eV] |
|---|---|---|---|---|
| a Estimated from the absorption onset.b Estimated from the highest energy vibronic transition in toluene at 77 K.c Shoulder. | ||||
| 3a | 392/326/338/366(shc) | 454 | 3.04 | 2.65 |
| 3b | 292/327/339/364 | 454.5 | 3.06 | 2.56 |
| 3c | 293/323/337/374 | 471.5 | 2.96 | 2.53 |
| 3d | 297/333/337/368(shc) | 456 | 3.04 | 2.57 |
| 3e | 288/310/323/360 | 451.5 | 3.05 | 2.87 |
| 3ai | 292/326/338/386 | 486.5 | 2.86 | 2.60 |
| 3bi | 292/326/339/386 | 486.5 | 2.85 | 2.51 |
| 3ci | 293/324/337/397 | 495.5 | 2.78 | 2.49 |
| 3di | 297/333/346/386 | 487.5 | 2.85 | 2.51 |
| 3ei | 286/308/323/367/389(shc) | 482 | 2.87 | 2.66 |
 | ||
| Fig. 3 Absorption spectra of 3b, 3c, 3d and 3bi (top) and normalized PL spectra of 3a, 3c, 3ai and 3ci (bottom). All spectra were recorded from 5 μM solution in DCM at r.t. | ||
In contrast to the photo-physical properties of all other investigated materials in this study, those of the ortho-derivatives 3c and 3ci are distinctly different, indicating the presence of electronic interactions between the carbazole and dithienophosphole moieties. Due to the fact that these interactions are absent in para-linked 3a and 3ai, which exhibit the highest degree of conjugation between the two molecular subunits, electronic exchange via the phenylene linker can be ruled out. However, the close vicinity of the planar carbazole and dithienophosphole, which has been confirmed by NOE measurements, suggests through-space interaction of the aromatic groups that are also revealed by the theoretical calculations (vide infra).
Bipolar organic materials exhibiting limited ICT have received great attention in recent years due to potential applications as host materials for transition metal complexes in PhOLEDs.39,53 The incorporation of phosphorescent triplet emitters in electro-optical devices overcomes the limitation of purely fluorescent emitter and theoretically allows for 100% internal quantum efficiency.54 These phosphorescent emitters have to be dispersed in an organic matrix for efficiency reasons. One major requirement of such host materials are high triplet energy (ET) values in order to confine the excited states on the emitter. However, the combination of donor and acceptor subunits within one molecule lowers the ET via ICT.39,53 Therefore, research focuses on the design and synthesis of donor–acceptor materials with decreased interaction between the molecular subunits. In this regard we investigated the ETs of the developed materials. The ETs were determined in frozen (solid) toluene solutions at 77 K from the highest vibronic transition of the delayed emission, and decrease in the order of para (3a = 2.65 eV/3ai = 2.60 eV) > meta (3b = 2.56 eV/3bi = 2.51 eV) > ortho (3c = 2.53 eV/3ci = 2.49 eV), respectively. These values are sufficiently high for applications as host materials in green and red PhOLEDs.39 While the additional t-butyl substituents at the carbazoles in 3d and 3di do not influence the energy levels, all of the n-butyl-substituted compounds feature slightly decreased ETs compared to the unsubstituted dithienophospholes. However, the influence of the n-butyl substituents on the ETs is lower compared to the corresponding optical bandgap ES (Fig. 4). Notably, the ICz-substituted compound 3e features a significantly higher ET of 2.87 eV (Fig. 5), rendering the application in blue PhOLEDs possible. In contrast to all other materials 3e exhibits vibronically well resolved phosphorescence (Fig. 5, ESI†), indicating a localized T1 (3LE) state.55 The transition from a charge transfer T1 (3CT) state to a 3LE in case of 3e can be explained by the weaker donor strength of the ICz moiety destabilizing the 3CT and thus might be the explanation for the significantly increased ET of 3e. In addition, the low singlet–triplet splitting of 0.18 eV makes this material particularly interesting. Recently, Adachi and coworkers introduced bipolar compounds with low singlet–triplet splitting as highly efficient electro-optical materials by means of thermally activated delayed fluorescence (TADF) due to thermal up-conversion of excited triplet states to singlet states.56 Thus, the investigated approach of attaching exocyclic donors to dithienophosphole oxides via the pyramidal coordinated phosphorus may provide a new design concept for efficient TADF materials.
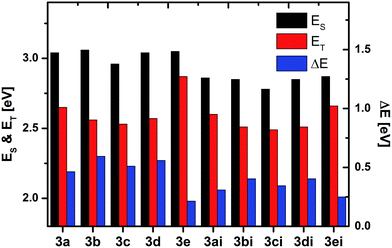 | ||
| Fig. 4 Singlet energies (ES), triplet energies (ET) and singlet–triplet splitting of all synthesized materials. | ||
2.3 Theoretical calculations
In order to provide some deeper understanding of the experimentally determined photo-physics of the materials in this study, we have performed Density-Functional Theory (DFT) calculations at the B3LYP/6-31+G(d) level of theory using the Gaussian 09 suite of programs.57 In order to save computing time, the butyl groups in the ‘i’ series of compounds were replaced with methyl substituents and the corresponding species are denominated as 3(a–e)i′. The DFT data generally support the experimentally determined features, with most compounds showing similar photo-physics, and the ortho-carbazole substituted species being distinct from their congeners. As a common denominator, the LUMO orbitals of all investigated species comprise the π*-system of the dithienophosphole scaffold, with the presence of the n-butyl groups being reflected in commonly increased energy levels by approximately 0.15 eV compared to their H-substituted relatives (Table 2). Another common denominator is the fact that the HOMO, HOMO−1 and HOMO−2 levels of all compounds are fairly close in energy for each compound (ΔE ∼ 0.4 eV) and consequently relevant for the observed experimental photo-physics. These orbitals respectively represent – to varying extents – the π-systems of the dithienophosphole and the triaryl amine scaffolds, as well as the π-bridge between the units in 3a,b,d,e and 3(a,b,d,e)i′ (ESI†). It should also be mentioned in this context that the HOMO for 3a,b,d and 3(a,b,d)i′ represents the π-system of the carbazole substituents with largely comparable energy levels (EHOMO ∼ −5.7 eV), a feature that has been confirmed by cyclic voltammetric (CV) measurements (Table 2).| Compd | EHOMO−2 [eV] (character) | EHOMO−1 [eV] (character) | EHOMO [eV] (character) | ELUMO [eV] (character) | Eox [V] CV | EHOMO [eV] CV |
|---|---|---|---|---|---|---|
| a S2P: dithienophosphole; cbz: carbazole; ph: phenylene; ICz: indolocarbazole; Eox: oxidation potential relative to Fc/Fc+. | ||||||
| 3a | −6.14 (π-S2P) | −6.13 (π-cbz) | −5.76 (π-cbz–ph) | −2.24 (π*-S2P) | 0.91 | −5.71 |
| 3b | −6.13 (π-S2P) | −6.12 (π-cbz) | −5.75 (π-cbz–ph) | −2.24 (π*-S2P) | 0.90 | −5.70 |
| 3c (syn) | −6.10 (π-S2P–cbz) | −5.93 (π-S2P–cbz) | −5.78 (π-S2P–cbz) | −2.08 (π*-S2P) | 0.86 | −5.66 |
| 3d | −6.10 (π-S2P) | −5.96 (π-cbz) | −5.55 (π-cbz–ph) | −2.21 (π*-S2P) | 0.74 | −5.54 |
| 3e | −6.47 (π-ICz) | −6.11 (π-S2P–ICz) | −5.92 (π-S2P–ICz) | −2.09 (π*-S2P) | 0.96 | −5.76 |
| 3ai | −6.10 (π-cbz) | −5.82 (π-S2P–cbz) | −5.69 (π-S2P–cbz) | −2.09 (π*-S2P) | 0.94 | −5.74 |
| 3bi | −6.09 (π-cbz) | −5.78 (π-S2P) | −5.72 (π-cbz–ph) | −2.09 (π*-S2P) | 0.88 | −5.68 |
| 3ci′-syn | −6.05 (π-cbz) | −5.80 (π-S2P–cbz) | −5.55 (π-S2P–cbz) | −1.93 (π*-S2P) | 0.85 | −5.65 |
| 3ci′-anti | −5.93 (π-cbz) | −5.72 (π-S2P–cbz) | −5.67 (π-S2P–cbz) | −2.08 (π*-S2P) | — | — |
| 3di | −5.93 (π-cbz) | −5.75 (π-S2P) | −5.51 (π-cbz–ph) | −2.06 (π*-S2P) | 0.73 | −5.53 |
| 3ei | −6.41 (π-ICz) | −6.02 (π-ICz) | −5.61 (π-S2P) | −1.95 (π*-S2P) | 0.78 | −5.58 |
Notably, the presence of the butyl substituents in the 2- and 6-positions of the dithienophospholes raises the energy of this unit's π-system, which leads to a switch in the orbital order with the π-system of the corresponding carbazole unit. The calculations suggest that the raised level of the dithienophosphole π-system in the para- and meta-carbazole species (HOMO−1, instead of HOMO−2 in 3a,b,d), in combination with the lowered LUMO levels is responsible for the red-shifted photo-physics of the ‘i’-series of compounds. This also indicates a reduced electronic communication between the two sub-chromophores. The effect of the t-butyl substituents in 3d and 3di′ is reflected in the increased energies of the respective HOMO levels at EHOMO ∼ −5.5 eV (CV: 3d: −5.54 eV; 3di: −5.53 eV).
In the case of 3e and 3ei′, the observed slight blue shift is the result of increased LUMO levels, compared to those of 3a,b,d(i′). However, the donor-effect of the methyl substituent is evident in the respective HOMO levels (−5.92 eV for 3e vs. −5.61 eV for 3ei′). This particular electrochemical behavior has been confirmed by CV measurements. In contrast to all other pairs of molecules the aliphatic substituent at the dithienophosphole leads to an increase of the HOMO energy in case of 3ei (−5.58 eV) compared to 3e (−5.76 eV). Remarkably, the orbital sequence and electronic contributions to the frontier orbitals of the indolocarbazole-substituted species (particularly for 3ei), approach those of the PAH-substituted species,30 clearly reflecting the effect of the diminishing donor-strength of the exocyclic substituent. The DFT data for the latter suggest only limited ICT present in this system, which is in fact absent in the PAH-relatives.
As mentioned above, the photo-physics of the ortho-substituted congeners were found to be distinct from those of the rest of the series. For this reason we have performed some more detailed studies on these species, including time-dependent (TD) DFT calculations at the B3LYP/6-31+G(d) level of theory. To establish the presence of electronic through-space interactions, we have included a conformer of 3ci′, in which the two sub-chromophores exhibit an anti-configuration, without the possibility of through-space interactions. In fact, the DFT calculations for 3ci′-anti provide orbital energies, that are not much different from those of the para- and meta-linked relatives, albeit with the HOMO largely comprising the dithienophosphole π-system (with the addition of the phenylene bridge), and the HOMO−1 and HOMO−2 showing increasing contribution for the carbazole π-system with diminishing contribution from the dithienophosphole scaffold (Fig. 6).
 | ||
| Fig. 6 TD-DFT calculation data for 3c, 3ci′-syn and 3ci′-anti showing notable transitions as well as their intensity (f = oscillator strength). Thickness of arrow indicates weight to transition. | ||
However, while there is certainly also a resemblance between the shape/contributions of these orbitals in 3ci′-anti with those of the two syn-configured conformers 3c and 3ci′-syn, the close proximity of the dithienophosphole and carbazole π-systems opens up through-space interactions in the latter. This is already reflected in orbital shapes and energies that deviate by about 0.1–0.2 eV from those of the other relatives (ESI, Table S2†). However, a much clearer picture is provided by the relevant transitions obtained from the TD-DFT calculations that show distinct differences between the syn- and anti-conformers (Fig. 6). For 3ci′-anti the lowest energy absorption, corresponding to an excitation HOMO → LUMO occurs at 352 nm, however with very low oscillator strength (f = 0.0029). Other transitions of note include HOMO → LUMO+1 (340 nm; f = 0.1201), HOMO−1 → LUMO (337 nm; f = 0.0722), with the most intense transition at 295 nm (f = 0.1744) corresponding to HOMO−1 → LUMO+1 (the π*-system of the phenylene bridge). In the case of the syn-conformers 3c and 3ci′-syn, the lowest energy transitions appear around 400 nm, in line with the experimental data, representing a mix of HOMO → LUMO (95%) and HOMO−1 → LUMO (5%) (f = 0.0154) for 3c and HOMO → LUMO (2%) and HOMO−1 → LUMO (96%) (f = 0.1133) for 3ci′-syn. Both species show two further transitions of note, respectively, that appear at 375 nm for 3c (HOMO−1 → LUMO (92%), HOMO → LUMO (4%); f = 0.0608), 380 nm for 3ci′-syn (HOMO−1 → LUMO; f = 0.0208), as well as 358.5 nm for 3c (HOMO−2 → LUMO; f = 0.0313) and 350 nm for 3ci′-syn (HOMO−2 → LUMO; f = 0.0121). These calculations are in line with the experimental data and rule out the anti-conformation to be relevant for the observed photo-physics and support the presence of through-space interactions resulting from syn-configuration.
3 Experimental section
3.1 General information
All reactions were carried out under nitrogen atmosphere, employing standard Schlenk techniques. Reagents and solvents were purchased from commercial suppliers and used without further purification unless noted otherwise. Anhydrous solvents were absolutized by an MBraun solvent purification system prior to use. NMR spectra were recorded on Bruker Avance-II/-III 400 MHz Spectrometers; for compounds 3c and 3ci 2D NMR spectra for complete signal assignment (COSY, HSQC, HMBC) as well as 1D and 2D NOESY spectra were obtained on a Bruker Avance IIIHD 600 MHz spectrometer equipped with a Prodigy BBO cryo probe. A Thermo Scientific LTQ Orbitrap XL hybrid FTMS (Fourier Transform Mass Spectrometer) equipped with a Thermo Fischer Exactive Plus Orbitrap (LC-ESI+) and a Shimadzu IT-TOF Mass Spectrometer were used for high resolution mass spectrometry. UV/Vis absorption and fluorescence emission spectra were recorded in DCM solutions (5 μM) with a Perkin Elmer Lambda 750 spectrometer and an Edinburgh FLS920, respectively. Time resolved experiments were obtained using a Quantel Brilliant tripled Nd-YAG laser (355 nm, 20 Hz repetition rate, pulse width ∼ 5 ns). Spectra were measured using a SPEX 270 monochromator equipped with both photomultiplier and CCD. This set-up is controlled using a home-built Labview-based program which allows using different instruments such as photon counting, oscilloscope, and additional mechanical shutters. For the measurement of the triplet emission, a mechanical shutter was triggered by the pulsed laser. A pretrigger period of 0.5 ms was followed by a 1 ms aperture and a rest time of 300–500 ms allowed obtaining the measurements shown in the ESI.† The slit of the monochromator was also opened further (up to 0.5 mm) to measure the triplet emission. Cyclic voltammetry was performed using a three electrode configuration consisting of a Pt working electrode, a Pt counter electrode and an Ag/AgCl reference electrode and a PGSTAT128N, ADC164, DAC164, External, DI048 potentiostat provided by Metrohm Autolab B.V with ferrocenium–ferrocene (Fc/Fc+) as standard. Measurements were carried out in a 0.5 mM solution in anhydrous DCM with Bu4NBF4 (0.1 M) as supporting electrolyte. The solutions were purged with nitrogen for 15 minutes prior to measurement. HOMO energy levels were calculated from the onset of the oxidation peaks. The onset potential was determined by the intersection of two tangents drawn at the background and the rising of the oxidation peaks. HOMO levels were calculated according to the equation HOMO = −(4.8 + Eox), where Eox is the oxidation potential relative to Fc/Fc+.3.2 Synthetic details
Dibrominated bithiophenes 2 (ref. 58) and 2i (ref. 31) were synthesized as described in literature. Dichlorophosphanes 1a–e were prepared in analogy to a previously published procedure from the corresponding brominated precursors and were used crude without any further purification.294-(4-(9H-Carbazol-9-yl)phenyl)-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3a). Starting from 2 (1.00 g, 3.1 mmol) and TMEDA (0.94 mL) in diethylether, n-BuLi (2.47 mL, 2.5 M), 1a (0.75 g, 2.2 mmol, 0.71 eq.) and H2O2 (30%, 2 mL) product 3a (130 mg, 0.29 mmol, 13%) was obtained as light yellowish solid after column chromatography (silica, ethyl acetate); undefined impurities (approx. 5–10%) were not separable from 3a by repeated column chromatography. 31P {1H} NMR (162 MHz, CD2Cl2): δ = 16.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.14 (d, J = 7.6 Hz, 2H), 7.95 (dd, J = 12.7, 8.3 Hz, 2H), 7.68 (dd, J = 8.3, 2.4 Hz, 2H), 7.47–7.39 (m, 6H), 7.32–7.25 (m, 4H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.5 (d, JCP = 24.3 Hz), 142.1 (d, JCP = 3.5 Hz), 140.8 (s), 139.4 (d, JCP = 112.3 Hz), 133.2 (d, JCP = 12.1 Hz), 129.4(4) (d, JCP = 108.1 Hz), 129.4(1) (d, JCP = 14.9 Hz), 127.5 (d, JCP = 13.0 Hz), 126.7 (s), 126.4 (d, JCP = 14.5 Hz), 124.2 (s), 121.0 (s), 120.9 (s), 110.3 (s) ppm. Calculated: m/z 453.04054 [M]+, 454.04837 [M + H]+, 476.03031 [M + Na]+. Found: MS (ESI): m/z 453.03978 [M]+, 454.04737 [M + H]+, 476.02944 [M + Na]+.
4-(4-(9H-Carbazol-9-yl)phenyl)-2,6-dibutyl-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3ai). Starting from 2i (0.80 g, 1.8 mmol) and TMEDA (0.58 mL) in diethylether, n-BuLi (1.46 mL, 2.5 M), 1a (0.63 g, 1.8 mmol) and H2O2 (30%, 2 mL) product 3ai (375 mg, 0.66 mmol, 36%) was obtained as yellowish solid after column chromatography (silica, ethyl acetate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 1
hexanes = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.14 (d, J = 7.7 Hz, 2H), 7.94 (dd, J = 12.7, 8.3 Hz, 2H), 7.68 (dd, J = 8.4, 2.3 Hz, 2H), 7.48–7.39 (m, 4H), 7.30 (ddd, J = 7.4, 7.4, 1.1 Hz, 2H), 6.91 (d, J = 2.0 Hz, 2H), 2.85 (t, J = 7.6 Hz, 4H), 1.69 (tt, J = 7.6, 7.6 Hz, 4H), 1.42 (qt, J = 7.6, 7.3 Hz, 4H), 0.95 (t, J = 7.3 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.8 (d, JCP = 14.4 Hz), 144.4 (d, JCP = 24.6 Hz), 141.9 (d, JCP = 3.2 Hz), 140.8 (s), 137.6 (d, JCP = 112.7 Hz), 133.2 (d, JCP = 12.3 Hz), 130.2 (d, JCP = 107.3 Hz), 127.4 (d, JCP = 13.0 Hz), 126.7 (s), 124.2 (s), 122.9 (d, JCP = 14.6 Hz), 121.0 (s), 120.9 (s), 110.3 (s), 34.2 (s), 30.6 (s), 22.7 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16518 [M]+, 566.17259 [M + H]+, 588.15436 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.14 (d, J = 7.7 Hz, 2H), 7.94 (dd, J = 12.7, 8.3 Hz, 2H), 7.68 (dd, J = 8.4, 2.3 Hz, 2H), 7.48–7.39 (m, 4H), 7.30 (ddd, J = 7.4, 7.4, 1.1 Hz, 2H), 6.91 (d, J = 2.0 Hz, 2H), 2.85 (t, J = 7.6 Hz, 4H), 1.69 (tt, J = 7.6, 7.6 Hz, 4H), 1.42 (qt, J = 7.6, 7.3 Hz, 4H), 0.95 (t, J = 7.3 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.8 (d, JCP = 14.4 Hz), 144.4 (d, JCP = 24.6 Hz), 141.9 (d, JCP = 3.2 Hz), 140.8 (s), 137.6 (d, JCP = 112.7 Hz), 133.2 (d, JCP = 12.3 Hz), 130.2 (d, JCP = 107.3 Hz), 127.4 (d, JCP = 13.0 Hz), 126.7 (s), 124.2 (s), 122.9 (d, JCP = 14.6 Hz), 121.0 (s), 120.9 (s), 110.3 (s), 34.2 (s), 30.6 (s), 22.7 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16518 [M]+, 566.17259 [M + H]+, 588.15436 [M + Na]+.
4-(3-(9H-Carbazol-9-yl)phenyl)-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3b). Starting from 2 (1.41 g, 4.4 mmol) in diethylether, n-BuLi (3.49 mL, 2.5 M), 1b (1.50 g, 4.4 mmol) and H2O2 (30%, 2 mL) product 3b (0.81 g, 1.8 mmol, 41%) was obtained as light yellowish solid after column chromatography (silica, ethyl acetate). 31P {1H} NMR (162 MHz, CDCl3): δ = 17.8 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.12 (d, J = 7.8 Hz, 2H), 7.89–7.81 (m, 2H), 7.79–7.75 (m, 1H), 7.73–7.68 (m, 1H), 7.41–7.36 (m, 4H), 7.32–7.23 (m, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.5 (d, JCP = 24.5 Hz), 140.9 (s), 139.3 (d, JCP = 112.7 Hz), 138.9 (d, JCP = 15.7 Hz), 133.3 (d, JCP = 105.8 Hz), 131.2 (d, JCP = 14.0 Hz), 131.1 (d, JCP = 2.7 Hz), 130.2 (d, JCP = 10.3 Hz), 129.5 (d, JCP = 15.1 Hz), 129.2 (d, JCP = 12.2 Hz), 126.6 (s), 126.3 (d, JCP = 14.6 Hz), 124.0 (s), 120.8(8) (s), 120.8(5) (s), 110.0 (s) ppm. Calculated: m/z 453.04054 [M]+, 454.04837 [M + H]+, 476.03031 [M + Na]+. Found: MS (ESI): m/z 453.04004 [M]+, 454.04705 [M + H]+, 476.02881 [M + Na]+.
4-(3-(9H-Carbazol-9-yl)phenyl)-2,6-dibutyl-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3bi). Starting from 2i (1.39 g, 3.2 mmol) in diethylether, n-BuLi (2.56 mL, 2.5 M), 1b (1.10 g, 3.2 mmol) and H2O2 (30%, 2 mL) product 3bi (0.55 g, 1.0 mmol, 30%) was obtained as light yellowish solid after column chromatography (silica, ethyl acetate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 1
hexanes = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.1 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 7.3 Hz, 2H), 7.91–7.67 (m, 4H), 7.41–7.35 (m, 2H), 7.33–7.26 (m, 4H) 6.90–6.88 (m, 2H), 2.84–2.79 (m, 4H), 1.70–1.61 (m, 4H), 1.44–1.34 (m, 4H), 0.94–0.89 (m, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.9 (d, JCP = 14.4 Hz), 144.4 (d, JCP = 24.5 Hz), 140.9 (s), 138.8 (d, JCP = 15.3 Hz), 137.4 (d, JCP = 112.8 Hz), 133.9 (d, JCP = 104.3 Hz), 131.1 (d, JCP = 13.8 Hz), 130.8 (d, JCP = 2.6 Hz), 130.2 (d, JCP = 10.3 Hz), 129.1 (d, JCP = 14.6 Hz), 126.6 (s), 124.0 (s), 122.7 (d, JCP = 14.6 Hz), 120.8 (s), 120.8 (s), 110.0 (s), 34.2 (s), 30.6 (s), 22.6 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16499 [M]+, 566.17241 [M + H]+, 588.15411 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.1 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 7.3 Hz, 2H), 7.91–7.67 (m, 4H), 7.41–7.35 (m, 2H), 7.33–7.26 (m, 4H) 6.90–6.88 (m, 2H), 2.84–2.79 (m, 4H), 1.70–1.61 (m, 4H), 1.44–1.34 (m, 4H), 0.94–0.89 (m, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.9 (d, JCP = 14.4 Hz), 144.4 (d, JCP = 24.5 Hz), 140.9 (s), 138.8 (d, JCP = 15.3 Hz), 137.4 (d, JCP = 112.8 Hz), 133.9 (d, JCP = 104.3 Hz), 131.1 (d, JCP = 13.8 Hz), 130.8 (d, JCP = 2.6 Hz), 130.2 (d, JCP = 10.3 Hz), 129.1 (d, JCP = 14.6 Hz), 126.6 (s), 124.0 (s), 122.7 (d, JCP = 14.6 Hz), 120.8 (s), 120.8 (s), 110.0 (s), 34.2 (s), 30.6 (s), 22.6 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16499 [M]+, 566.17241 [M + H]+, 588.15411 [M + Na]+.
4-(2-(9H-Carbazol-9-yl)phenyl)-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3c). Starting from 2 (1.00 g, 3.1 mmol) and TMEDA (0.94 mL) in diethylether, n-BuLi (2.47 mL, 2.5 M), 1c (1.06 g, 3.1 mmol) and H2O2 (30%, 1.5 mL) product 3c (353 mg, 0.78 mmol, 25%) was obtained as light yellowish solid after column chromatography (silica, ethyl acetate). 31P {1H} NMR (162 MHz, CDCl3): δ = 12.9 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.76 (ddd, J = 13.2, 7.8, 1.7 Hz, 1H), 7.82–7.83 (m, 3H), 7.81–7.69 (m, 1H), 7.24–7.20 (m, 1H), 7.13–7.04 (m, 4H), 6.75 (dd, J = 4.9, 3.5 Hz, 2H), 6.68 (dd, J = 4.9, 2.6 Hz, 2H), 6.44 (d, J = 7.84 Hz, 2H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 145.8 (d, JCP = 25.5 Hz), 142.4 (s), 138.9 (d, JCP = 6.1 Hz), 137.2 (d, JCP = 115.6 Hz), 137.2 (d, JCP = 7.3 Hz), 134.6 (d, JCP = 2.3 Hz), 132.8 (d, JCP = 101.8 Hz), 132.0 (d, JCP = 7.7 Hz), 130.4 (d, JCP = 11.3 Hz), 128.3 (d, JCP = 15.3 Hz), 126.1 (s), 125.4 (d, JCP = 15.3 Hz), 123.4 (s), 120.2 (s), 120.0 (s), 110.2 (s) ppm. Calculated: m/z 453.04054 [M]+, 454.04837 [M + H]+, 476.03031 [M + Na]+. Found: MS (ESI): m/z 453.03945 [M]+, 454.04731 [M + H]+, 476.02871 [M + Na]+.
4-(2-(9H-Carbazol-9-yl)phenyl)-2,6-dibutyl-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3ci). Starting from 2i (1.00 g, 2.3 mmol) and TMEDA (0.69 mL) in THF, n-BuLi (1.83 mL, 2.5 M), 1c (0.79 g, 2.3 mmol) and H2O2 (30%, 1.5 mL) product 3ci (220 mg, 0.39 mmol, 17%) was obtained as light yellowish solid after column chromatography (silica, ethyl acetate
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexanes = 1
hexanes = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 14.1 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.70 (ddd, J = 13.2, 7.9, 1.5 Hz, 1H), 7.90–7.81 (m, 3H), 7.75–7.73 (m, 1H), 7.18 (dd, J = 6.8, 5.9 Hz, 1H), 7.14–7.08 (m, 4H), 6.49–6.45 (m, 2H), 6.34 (d, J = 2.2 Hz, 2H), 2.55–2.42 (m, 4H), 1.53–1.44 (m, 4H), 1.34 (qt, J = 7.5, 7.5 Hz, 4H), 0.92 (t, J = 7.5 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 149.6 (d, JCP = 14.8 Hz), 144.0 (d, JCP = 25.3 Hz), 142.5 (s), 138.9 (d, JCP = 5.4 Hz), 137.1 (d, JCP = 6.9 Hz), 135.9 (d, JCP = 116.0 Hz), 134.3 (d, JCP = 2.3 Hz), 133.5 (d, JCP = 100.3 Hz), 131.8 (d, JCP = 7.7 Hz), 130.3 (d, JCP = 11.0 Hz), 125.7 (s), 123.5 (s), 122.1 (d, JCP = 14.6 Hz), 120.1 (s), 120.0 (s), 110.5 (s) 33.7 (s), 30.3 (s), 22.8 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16504 [M]+, 566.17275 [M + H]+, 588.15397 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 14.1 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.70 (ddd, J = 13.2, 7.9, 1.5 Hz, 1H), 7.90–7.81 (m, 3H), 7.75–7.73 (m, 1H), 7.18 (dd, J = 6.8, 5.9 Hz, 1H), 7.14–7.08 (m, 4H), 6.49–6.45 (m, 2H), 6.34 (d, J = 2.2 Hz, 2H), 2.55–2.42 (m, 4H), 1.53–1.44 (m, 4H), 1.34 (qt, J = 7.5, 7.5 Hz, 4H), 0.92 (t, J = 7.5 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 149.6 (d, JCP = 14.8 Hz), 144.0 (d, JCP = 25.3 Hz), 142.5 (s), 138.9 (d, JCP = 5.4 Hz), 137.1 (d, JCP = 6.9 Hz), 135.9 (d, JCP = 116.0 Hz), 134.3 (d, JCP = 2.3 Hz), 133.5 (d, JCP = 100.3 Hz), 131.8 (d, JCP = 7.7 Hz), 130.3 (d, JCP = 11.0 Hz), 125.7 (s), 123.5 (s), 122.1 (d, JCP = 14.6 Hz), 120.1 (s), 120.0 (s), 110.5 (s) 33.7 (s), 30.3 (s), 22.8 (s), 14.1 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16504 [M]+, 566.17275 [M + H]+, 588.15397 [M + Na]+.
4-(3-(3,6-Di-tert-butyl-9H-carbazol-9-yl)phenyl)-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3d). Starting from 2 (0.62 g, 1.9 mmol) and TMEDA (0.58 mL) in THF, n-BuLi (1.53 mL, 2.5 M), 1d (0.87 g, 1.9 mmol) and H2O2 (30%, 1 mL) product 3d (185 mg, 0.33 mmol, 17%) was obtained as light yellowish solid after column chromatography (silica, DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN = 20
MeCN = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 18.0 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 1.9 Hz, 2H), 7.86–7.74 (m, 3H), 7.68 (ddd, J = 7.7, 7.7, 3.7 Hz, 1H), 7.44 (dd, J = 8.7, 1.8 Hz, 2H), 7.38 (dd, J = 4.9, 3.5 Hz, 2H), 7.26–7.22 (m, 4H), 1.45 (s, 18H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.6 (d, JCP = 24.1 Hz), 144.0 (s), 139.4 (d, JCP = 15.9 Hz), 139.2 (d, JCP = 112.7 Hz), 139.2 (s), 132.9 (d, JCP = 105.8 Hz), 131.1 (d, JCP = 14.0 Hz), 130.7 (d, JCP = 2.8 Hz), 129.6 (d, JCP = 10.4 Hz), 129.5 (d, JCP = 15.2 Hz), 128.8 (d, JCP = 12.2 Hz), 126.4 (d, JCP = 14.5 Hz), 124.3 (s), 124.0 (s), 116.9 (s), 109.4 (s), 35.2 (s), 32.3 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16500 [M]+, 566.17249 [M + H]+, 588.15648 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 18.0 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 1.9 Hz, 2H), 7.86–7.74 (m, 3H), 7.68 (ddd, J = 7.7, 7.7, 3.7 Hz, 1H), 7.44 (dd, J = 8.7, 1.8 Hz, 2H), 7.38 (dd, J = 4.9, 3.5 Hz, 2H), 7.26–7.22 (m, 4H), 1.45 (s, 18H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.6 (d, JCP = 24.1 Hz), 144.0 (s), 139.4 (d, JCP = 15.9 Hz), 139.2 (d, JCP = 112.7 Hz), 139.2 (s), 132.9 (d, JCP = 105.8 Hz), 131.1 (d, JCP = 14.0 Hz), 130.7 (d, JCP = 2.8 Hz), 129.6 (d, JCP = 10.4 Hz), 129.5 (d, JCP = 15.2 Hz), 128.8 (d, JCP = 12.2 Hz), 126.4 (d, JCP = 14.5 Hz), 124.3 (s), 124.0 (s), 116.9 (s), 109.4 (s), 35.2 (s), 32.3 (s) ppm. Calculated: m/z 565.16574 [M]+, 566.17357 [M + H]+, 588.15551 [M + Na]+. Found: MS (ESI): m/z 565.16500 [M]+, 566.17249 [M + H]+, 588.15648 [M + Na]+.
4-(3-(3,6-Di-tert-butyl-9H-carbazol-9-yl)phenyl)-2,6-dibutyl-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3di). Starting from 2i (0.83 g, 1.9 mmol) and TMEDA (0.58 mL) in THF, n-BuLi (1.53 mL, 2.5 M), 1d (0.87 g, 1.9 mmol) and H2O2 (30%, 1 mL) product 3di (0.47 g, 0.7 mmol, 36%) was obtained as light yellowish solid after column chromatography (silica, DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN = 20
MeCN = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 1.8 Hz, 2H), 7.88–7.73 (m, 3H), 7.68 (ddd, J = 7.6, 7.6, 3.5 Hz, 1H), 7.43 (dd, J = 8.7, 1.9 Hz, 2H), 7.25 (d, J = 8.6 Hz, 2H), 6.89–6.88 (m, 2H), 2.82 (t, J = 7.6 Hz, 4H), 1.67 (tt, J = 7.6, 7.6 Hz, 4H), 1.45–1.34 (m, 22H), 0.92 (t, J = 7.3 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.9 (d, JCP = 14.1 Hz), 144.5 (d, JCP = 24.5 Hz), 143.9 (s), 139.3 (d, JCP = 15.3 Hz), 139.2 (s), 137.3 (d, JCP = 112.6 Hz), 133.6 (d, JCP = 105.0 Hz), 131.0 (d, JCP = 13.9 Hz), 130.4 (d, JCP = 3.7 Hz), 129.7 (d, JCP = 10.1 Hz), 128.6 (d, JCP = 12.4 Hz), 124.3 (s), 124.0 (s), 122.7 (d, JCP = 13.8 Hz), 116.9 (s), 109.5 (s), 35.2 (s), 34.2 (s), 32.3 (s), 30.6 (s), 22.7 (s), 14.1 (s) ppm. Calculated: m/z 677.29094 [M]+, 678.29877 [M + H]+, 700.28071 [M + Na]+. Found: MS (ESI): m/z 677.28998 [M]+, 678.29816 [M + H]+, 700.27997 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 19.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.13 (d, J = 1.8 Hz, 2H), 7.88–7.73 (m, 3H), 7.68 (ddd, J = 7.6, 7.6, 3.5 Hz, 1H), 7.43 (dd, J = 8.7, 1.9 Hz, 2H), 7.25 (d, J = 8.6 Hz, 2H), 6.89–6.88 (m, 2H), 2.82 (t, J = 7.6 Hz, 4H), 1.67 (tt, J = 7.6, 7.6 Hz, 4H), 1.45–1.34 (m, 22H), 0.92 (t, J = 7.3 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.9 (d, JCP = 14.1 Hz), 144.5 (d, JCP = 24.5 Hz), 143.9 (s), 139.3 (d, JCP = 15.3 Hz), 139.2 (s), 137.3 (d, JCP = 112.6 Hz), 133.6 (d, JCP = 105.0 Hz), 131.0 (d, JCP = 13.9 Hz), 130.4 (d, JCP = 3.7 Hz), 129.7 (d, JCP = 10.1 Hz), 128.6 (d, JCP = 12.4 Hz), 124.3 (s), 124.0 (s), 122.7 (d, JCP = 13.8 Hz), 116.9 (s), 109.5 (s), 35.2 (s), 34.2 (s), 32.3 (s), 30.6 (s), 22.7 (s), 14.1 (s) ppm. Calculated: m/z 677.29094 [M]+, 678.29877 [M + H]+, 700.28071 [M + Na]+. Found: MS (ESI): m/z 677.28998 [M]+, 678.29816 [M + H]+, 700.27997 [M + Na]+.
4-(Indolo[3,2,1-jk]carbazol-2-yl)-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3e). Starting from 2 (0.50 g, 1.5 mmol) in THF, n-BuLi (1.22 mL, 2.5 M), 1e (0.52 g, 1.5 mmol) and H2O2 (30%, 2 mL) product 3e (73 mg, 0.16 mmol, 11%) was obtained as light yellowish solid after column chromatography (silica, DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN = 5
MeCN = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 22.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.46 (d, J = 13.0 Hz, 2H), 8.15 (d, J = 7.8 Hz, 2H), 7.95 (d, J = 7.9 Hz, 2H), 7.62 (dd, J = 7.7, 7.7 Hz, 2H), 7.43–7.36 (m, 4H), 7.20 (dd, J = 4.8, 2.3 Hz, 2H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.3 (d, JCP = 2.3 Hz), 146.2 (d, JCP = 23.6 Hz), 140.8 (d, JCP = 111.0 Hz), 139.7 (s), 129.8 (s), 129.2 (d, JCP = 14.6 Hz), 128.2 (s), 126.4 (d, JCP = 14.5 Hz), 124.1 (s), 123.9 (d, JCP = 108.1 Hz), 123.0 (s), 122.9 (d, JCP = 14.5 Hz), 119.5 (d, JCP = 17.5 Hz), 113.0 (s) ppm. Calculated: m/z 451.02489 [M]+, 452.03272 [M + H]+, 474.01466 [M + Na]+. Found: MS (ESI): m/z 451.02445 [M]+, 452.03177 [M + H]+, 474.01384 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 22.2 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.46 (d, J = 13.0 Hz, 2H), 8.15 (d, J = 7.8 Hz, 2H), 7.95 (d, J = 7.9 Hz, 2H), 7.62 (dd, J = 7.7, 7.7 Hz, 2H), 7.43–7.36 (m, 4H), 7.20 (dd, J = 4.8, 2.3 Hz, 2H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 146.3 (d, JCP = 2.3 Hz), 146.2 (d, JCP = 23.6 Hz), 140.8 (d, JCP = 111.0 Hz), 139.7 (s), 129.8 (s), 129.2 (d, JCP = 14.6 Hz), 128.2 (s), 126.4 (d, JCP = 14.5 Hz), 124.1 (s), 123.9 (d, JCP = 108.1 Hz), 123.0 (s), 122.9 (d, JCP = 14.5 Hz), 119.5 (d, JCP = 17.5 Hz), 113.0 (s) ppm. Calculated: m/z 451.02489 [M]+, 452.03272 [M + H]+, 474.01466 [M + Na]+. Found: MS (ESI): m/z 451.02445 [M]+, 452.03177 [M + H]+, 474.01384 [M + Na]+.
4-(Indolo[3,2,1-jk]carbazol-2-yl)-2,6-dibutyl-4H-phospholo[3,2-b:4,5-b′]dithiophene 4-oxide (3ei). Starting from 2i (0.97 g, 2.2 mmol) in THF, n-BuLi (1.78 mL, 2.5 M), 1e (0.76 g, 2.2 mmol) and H2O2 (30%, 2 mL) product 3ei (152 mg, 0.27 mmol, 12%) was obtained as light yellowish solid after column chromatography (silica, DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN = 20
MeCN = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 31P {1H} NMR (162 MHz, CDCl3): δ = 23.3 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.45 (d, J = 12.9 Hz, 2H), 8.15 (d, J = 7.9 Hz, 2H), 7.94 (d, J = 8.2 Hz, 2H), 7.61 (dd, J = 7.7, 7.7 Hz, 2H), 7.40 (dd, J = 7.7, 7.7 Hz, 2H), 6.85 (s, 2H), 2.82 (t, J = 7.5 Hz, 4H), 1.66 (tt, J = 7.5, 7.5 Hz, 4H), 1.39 (qt, J = 7.5, 7.4 Hz, 4H), 0.92 (t, J = 7.4 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.6 (d, JCP = 13.9 Hz), 146.3 (d, JCP = 2.3 Hz), 144.0 (d, JCP = 23.7 Hz), 139.7 (s), 138.9 (d, JCP = 111.2 Hz), 129.8 (s), 128.2 (s), 124.7 (d, JCP = 106.6 Hz), 124.1 (s), 123.0 (s), 122.9 (d, JCP = 14.6 Hz), 122.8 (d, JCP = 14.2 Hz), 119.4 (d, JCP = 16.9 Hz), 113.0 (s), 34.2 (s), 30.6 (s), 22.6 (s), 14.1 (s) ppm. Calculated: m/z 563.15009 [M]+, 564.15792 [M + H]+, 586.13986 [M + Na]+. Found: MS (ESI): m/z 563.14933 [M]+, 564.15704 [M + H]+, 586.13880 [M + Na]+.
1). 31P {1H} NMR (162 MHz, CDCl3): δ = 23.3 (s) ppm. 1H NMR (400 MHz, CD2Cl2): δ = 8.45 (d, J = 12.9 Hz, 2H), 8.15 (d, J = 7.9 Hz, 2H), 7.94 (d, J = 8.2 Hz, 2H), 7.61 (dd, J = 7.7, 7.7 Hz, 2H), 7.40 (dd, J = 7.7, 7.7 Hz, 2H), 6.85 (s, 2H), 2.82 (t, J = 7.5 Hz, 4H), 1.66 (tt, J = 7.5, 7.5 Hz, 4H), 1.39 (qt, J = 7.5, 7.4 Hz, 4H), 0.92 (t, J = 7.4 Hz, 6H) ppm. 13C {1H} NMR (100 MHz, CD2Cl2): δ = 150.6 (d, JCP = 13.9 Hz), 146.3 (d, JCP = 2.3 Hz), 144.0 (d, JCP = 23.7 Hz), 139.7 (s), 138.9 (d, JCP = 111.2 Hz), 129.8 (s), 128.2 (s), 124.7 (d, JCP = 106.6 Hz), 124.1 (s), 123.0 (s), 122.9 (d, JCP = 14.6 Hz), 122.8 (d, JCP = 14.2 Hz), 119.4 (d, JCP = 16.9 Hz), 113.0 (s), 34.2 (s), 30.6 (s), 22.6 (s), 14.1 (s) ppm. Calculated: m/z 563.15009 [M]+, 564.15792 [M + H]+, 586.13986 [M + Na]+. Found: MS (ESI): m/z 563.14933 [M]+, 564.15704 [M + H]+, 586.13880 [M + Na]+.
3.3 Computational details
Density-Functional-Theory (DFT) and Time-Dependent (TD) DFT calculations have been carried out at the B3LYP/6-31+G(d) level of theory using the GAUSSIAN 09 suite of programs.573.4 Single crystal diffraction
Crystals of 3c (ref. 49) suitable for single crystal diffraction were selected under a polarizing microscope, embedded in perfluorinated oil and attached to Kapton® mounts. Intensity data were collected in a dry stream of nitrogen at 100 K on a Bruker KAPPA APEX II diffractometer system. Since automatic unit-cell determination failed, reflection position were analyzed using the RLATT60 tool. Two monoclinic domains related by reflection at (101) could be identified. They were integrated concurrently using SAINT-Plus60 with overlap detection (HKLF5-style output file) and an empirical absorption correction using the multi-scan approach implemented in TWINABS60 was applied. The crystal structures were solved by charge-flipping implemented in SUPERFLIP61 and refined against F with the JANA2006 (ref. 62) software package. The non-H atoms were refined with anisotropic displacement parameters (ADPs). The H atoms were placed at calculated positions and refined as riding on the parent C atoms. More details on data collection and refinement are summarized in the ESI.†4 Conclusion
In summary, the synthesis and characterization of novel dithienophosphole oxide donor–acceptor materials, applying various phenylcarbazole and indolocarbazole derivatives, has been presented. The following major conclusion can be drawn:(1) The intramolecular charge transfer through the phosphorous atom can be virtually suppressed by reducing the donor strength in the donor–acceptor scaffold;
(2) Compounds potentially suitable as host materials in PhOLED applications could be obtained (triplet energies up to 2.87 eV; singlet–triplet splitting of 0.18 eV);
(3) Through space interactions have been established for the ortho-phenylcarbazole structure.
The theoretical calculations support the aforementioned assumptions drawn from the experimental data.
As a result, the approach of increasing the planarization of the triarylamine type donor in stepwise fashion and, thus, decreasing the donor strength clearly broadens the scope of potential applications of the dithienophosphole oxide scaffold.
Acknowledgements
T. B. thanks the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation for financial support. This work was supported in part by the Vienna University of Technology research funds, the Austrian Federal Ministry of Science, Research and Economy and the Swiss National Science Foundation. The X-ray centre of the Vienna University of Technology is acknowledged for providing access to the single-crystal diffractometer. K. Föttinger is acknowledged for assisting the photo-physical analysis. The student exchange of H. P. was financially supported by the Joint Study Grant (TASSEP for Canada, 2012) and the KUWI Grant (Vienna University of Technology, 2013).References
- S. Reineke, M. Thomschke, B. Lüssem and K. Leo, Rev. Mod. Phys., 2013, 85, 1245–1293 CrossRef CAS.
- M. Zhu and C. Yang, Chem. Soc. Rev., 2013, 42, 4963–4976 RSC.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. H. Li, R. F. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931–7958 CrossRef CAS PubMed.
- Q. Meng, H. L. Dong, W. P. Hu and D. B. Zhu, J. Mater. Chem., 2011, 21, 11708–11721 RSC.
- C. A. Di, F. J. Zhang and D. B. Zhu, Adv. Mater., 2013, 25, 313–330 CrossRef CAS PubMed.
- L. Torsi, M. Magliulo, K. Manoli and G. Palazzo, Chem. Soc. Rev., 2013, 42, 8612–8628 RSC.
- Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- A. Facchetti, Chem. Mater., 2011, 23, 733–758 CrossRef CAS.
- J. G. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. N. Bao, J. Am. Chem. Soc., 2013, 135, 6724–6746 CrossRef CAS PubMed.
- Z. M. Hudson and S. N. Wang, Acc. Chem. Res., 2009, 42, 1584–1596 CrossRef CAS PubMed.
- F. Jakle, Chem. Rev., 2010, 110, 3985–4022 CrossRef CAS PubMed.
- C. R. Wade, A. E. J. Broomsgrove, S. Aldridge and F. P. Gabbai, Chem. Rev., 2010, 110, 3958–3984 CrossRef CAS PubMed.
- J. W. Chen and Y. Cao, Macromol. Rapid Commun., 2007, 28, 1714–1742 CrossRef CAS.
- J. Ohshita, Macromol. Chem. Phys., 2009, 210, 1360–1370 CrossRef CAS.
- B. C. Schroeder, Z. G. Huang, R. S. Ashraf, J. Smith, P. D'Angelo, S. E. Watkins, T. D. Anthopoulos, J. R. Durrant and I. McCulloch, Adv. Funct. Mater., 2012, 22, 1663–1670 CrossRef CAS.
- X. M. He and T. Baumgartner, RSC Adv., 2013, 3, 11334–11350 RSC.
- A. A. Jahnke and D. S. Seferos, Macromol. Rapid Commun., 2011, 32, 943–951 CrossRef CAS PubMed.
- T. Baumgartner and R. Reau, Chem. Rev., 2006, 106, 4681–4727 CrossRef CAS PubMed.
- J. Crassous and R. Reau, Dalton Trans., 2008, 6865–6876 RSC.
- Y. Matano and H. Imahoria, Org. Biomol. Chem., 2009, 7, 1258–1271 CAS.
- S. O. Jeon and J. Y. Lee, J. Mater. Chem., 2012, 22, 4233–4243 RSC.
- Y. Ren and T. Baumgartner, Dalton Trans., 2012, 41, 7792–7800 RSC.
- C. Romero-Nieto and T. Baumgartner, Synlett, 2013, 24, 920–937 CrossRef CAS.
- T. Baumgartner, Acc. Chem. Res., 2014, 47, 1613–1622 CrossRef CAS PubMed.
- M. G. Hobbs and T. Baumgartner, Eur. J. Inorg. Chem., 2007, 3611–3628 CrossRef CAS.
- T. Baumgartner, W. Bergmans, T. Karpati, T. Neumann, M. Nieger and L. Nyulaszi, Chem.–Eur. J., 2005, 11, 4687–4699 CrossRef CAS PubMed.
- Y. Dienes, S. Durben, T. Karpati, T. Neumann, U. Englert, L. Nyulaszi and T. Baumgartner, Chem.–Eur. J., 2007, 13, 7487–7500 CrossRef CAS PubMed.
- M. Stolar and T. Baumgartner, New J. Chem., 2012, 36, 1153–1160 RSC.
- C. J. Chua, Y. Ren and T. Baumgartner, Org. Lett., 2012, 14, 1588–1591 CrossRef CAS PubMed.
- C. J. Chua, Y. Ren and T. Baumgartner, Organometallics, 2012, 31, 2425–2436 CrossRef CAS.
- C. J. Chua, Y. Ren, M. Stolar, S. Xing, T. Linder and T. Baumgartner, Eur. J. Inorg. Chem., 2014, 1767–1774 CrossRef CAS.
- T. Baumgartner, T. Neumann and B. Wirges, Angew. Chem., Int. Ed., 2004, 43, 6197–6201 CrossRef CAS PubMed.
- S. Durben, Y. Dienes and T. Baumgartner, Org. Lett., 2006, 8, 5893–5896 CrossRef CAS PubMed.
- C. Romero-Nieto, S. Durben, I. M. Kormos and T. Baumgartner, Adv. Funct. Mater., 2009, 19, 3625–3631 CrossRef CAS.
- C. Romero-Nieto, K. Kamada, D. T. Cramb, S. Merino, J. Rodriguez-Lopez and T. Baumgartner, Eur. J. Inorg. Chem., 2010, 5225–5231 CrossRef CAS.
- C. Romero-Nieto, M. Marcos, S. Merino, J. Barbera, T. Baumgartner and J. Rodriguez-Lopez, Adv. Funct. Mater., 2011, 21, 4088–4099 CrossRef CAS.
- Y. Ren, W. H. Kan, V. Thangadurai and T. Baumgartner, Angew. Chem., Int. Ed., 2012, 51, 3964–3968 CrossRef CAS PubMed.
- G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245–1330 CrossRef CAS PubMed.
- Y. Tao, C. Yang and J. Qin, Chem. Soc. Rev., 2011, 40, 2943–2970 RSC.
- A. Mishra and P. Bäuerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS PubMed.
- X. Cai, A. B. Padmaperuma, L. S. Sapochak, P. A. Vecchi and P. E. Burrows, Appl. Phys. Lett., 2008, 92, 083308 CrossRef.
- S. O. Jeon, K. S. Yook, C. W. Joo and J. Y. Lee, Adv. Funct. Mater., 2009, 19, 3644–3649 CrossRef CAS.
- H.-H. Chou and C.-H. Cheng, Adv. Mater., 2010, 22, 2468–2471 CrossRef CAS PubMed.
- P. K. Koech, E. Polikarpov, J. E. Rainbolt, L. Cosimbescu, J. S. Swensen, A. L. von Ruden and A. B. Padmaperuma, Org. Lett., 2010, 12, 5534–5537 CrossRef CAS PubMed.
- U. S. Bhansali, E. Polikarpov, J. S. Swensen, W.-H. Chen, H. Jia, D. J. Gaspar, B. E. Gnade, A. B. Padmaperuma and M. A. Omary, Appl. Phys. Lett., 2009, 95, 233304 CrossRef.
- P. Kautny, Diploma thesis, Vienna University of Technology, 2013.
- E. Mondal, W.-Y. Hung, H.-C. Dai and K.-T. Wong, Adv. Funct. Mater., 2013, 23, 3096–3105 CrossRef CAS.
- (3c): C26H16NOPS2, Mr = 453.50, T = 100 K, monoclinic, space group P21, a = 8.3295(6) Å, b = 14.1464(10) Å, c = 8.8132(6) Å, β = 103.1530(19)°, V = 1011.24(12) Å3, Z = 2, ρcalcd = 1.4894 Mg m−3, μ = 0.363 mm−1, λ = 0.71070 Å, θmax = 32.72°, 82
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676 measured reflections, 7360 [R(int) = 0.0407] independent reflections, GOF on F = 1.92, R1 = 0.0198, wR2 = 0.0272 (I > 3σ(I)), R1 = 0.0201, wR2 = 0.0273 (all data), largest difference peak and hole 0.280 and −0.200 e Å−3. Twinned by twofold rotation about [10−1] with a 63.75
676 measured reflections, 7360 [R(int) = 0.0407] independent reflections, GOF on F = 1.92, R1 = 0.0198, wR2 = 0.0272 (I > 3σ(I)), R1 = 0.0201, wR2 = 0.0273 (all data), largest difference peak and hole 0.280 and −0.200 e Å−3. Twinned by twofold rotation about [10−1] with a 63.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36.25(7) volume ratio. CCDC reference number 1407916†.
36.25(7) volume ratio. CCDC reference number 1407916†. - H. H. Li, Y. Wang, K. Yuan, Y. Tao, R. F. Chen, C. Zheng, X. H. Zhou, J. F. Li and W. Huang, Chem. Commun., 2014, 50, 15760–15763 RSC.
- C. Romero-Nieto, S. Merino, J. Rodriguez-Lopez and T. Baumgartner, Chem.–Eur. J., 2009, 15, 4135–4145 CrossRef CAS PubMed.
- P. Kautny, D. Lumpi, Y. Wang, A. Tissot, J. Bintinger, E. Horkel, B. Stöger, C. Hametner, H. Hagemann, D. Ma and J. Fröhlich, J. Mater. Chem. C, 2014, 2, 2069–2081 RSC.
- A. Chaskar, H.-F. Chen and K.-T. Wong, Adv. Mater., 2011, 23, 3876–3895 CrossRef CAS PubMed.
- C. Adachi, M. A. Baldo, M. E. Thompson and S. R. Forrest, J. Appl. Phys., 2001, 90, 5048–5051 CrossRef CAS.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Nat. Photonics, 2014, 8, 326–332 CrossRef CAS.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- J. Ohshita, M. Nodono, H. Kai, T. Watanabe, A. Kunai, K. Komaguchi, M. Shiotani, A. Adachi, K. Okita, Y. Harima, K. Yamashita and M. Ishikawa, Organometallics, 1999, 18, 1453–1459 CrossRef CAS.
- Y. Ren, Y. Dienes, S. Hettel, M. Parvez, B. Hoge and T. Baumgartner, Organometallics, 2009, 28, 734–740 CrossRef CAS.
- SAINT and SADABS, Bruker Analytical X-ray Instruments, Inc., Madinson, WI, USA, 2008 Search PubMed.
- L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786–790 CrossRef CAS.
- V. Petříček, M. Dušek and L. Palatinus, Z. Kristallogr., 2014, 229, 345 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1407916. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra13651b |
| ‡ Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |



