MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes
Abstract
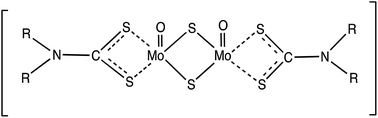
Due to the complexity of the processes, the degradation mechanisms of molybdenum dithiocarbamate (MoDTC)-containing oil are still not fully understood. In order to get a better understanding of how a MoDTC additive works at the molecular level, correlation between its chemical behaviour in a bulk oil during thermo-oxidative degradation and its ability to reduce friction has been investigated. The combination of High-Performance Liquid Chromatography (HPLC), Fourier Transform Infrared Spectroscopy (FT-IR) and Mass Spectroscopy (MS) techniques has provided much detailed information about the complex chemistry involved in the degradation process. Finally, the relationship between MoDTC additive depletion and its effectiveness in decreasing friction has been studied and a hypothesis on the chemical pathway followed by MoDTC during a thermo-oxidative degradation process has been proposed.

 Please wait while we load your content...
Please wait while we load your content...