Fabrication of Fe3O4@SiO2@RGO nanocomposites and their excellent absorption properties with low filler content†
Ya-Fei Pan,
Guang-Sheng Wang* and
Yong-Hai Yue*
Key Laboratory of Bio-Inspired Smart Interfacial Science and Technology of Ministry of Education, School of Chemistry and Environment, Beihang University, Beijing 100191, PR China. E-mail: wanggsh@buaa.edu.cn
First published on 17th August 2015
Abstract
Novel Fe3O4@SiO2@RGO nanocomposites were fabricated and the nanocomposites which were embedded into paraffin wax with low filler content (20 wt%) showed excellent microwave absorption properties. The minimum reflection loss can reach −26.6 dB at 9.7 GHz, and the reflection loss was less than −10 dB in the frequency range from 4.4 to 17.3 GHz with an absorber thickness of 2.0–5.0 mm. Compared with the pure Fe3O4 and Fe3O4@SiO2, the Fe3O4@SiO2@RGO nanocomposites exhibited superior absorption properties. The mechanism of enhanced wave absorption properties was explained.
Introduction
In recent years, with the fast development of wireless communication and the extensive utilization of electronic devices, electromagnetic interference has become a new environmental problem. Hence, much attention has focused on high-performance microwave absorption materials, which have prospective applications in reduction of electromagnetic radiation and improvement of electromagnetic interference shielding.1–3 Over the past decades, due to the strong absorption and large anisotropic energy, ferrites have attracted considerable attention to develop novel microwave absorption materials.4–6 Among the various ferrite materials, extensive studies have been done to develop the magnetite (Fe3O4) as microwave absorber, with the advantages of low cost and strong absorption.7As a typical double-complex medium with dielectric loss and magnetic loss, Fe3O4 filled as absorber can exhibit good microwave absorption property, which has been reported by S. Kolev et al.8 The minimum calculated reflection loss can reach −25 dB at 6.9 GHz with 35 wt% Fe3O4 when the matching thickness is 4 mm. Ni et al.9 prepared well-dispersed Fe3O4 nanocrystals and obtained the minimum reflection loss value of −21.2 dB at 8.2 GHz at the 30 wt% filler content. Wei et al.10 studied the microwave absorption properties of Fe3O4 magnetic films and the results showed that the matching frequency for reflection loss exceeded −20 dB at 13.0 GHz. Many researchers have concentrated on improving the microwave absorption performance, for example considering the impedance matching problem, the Fe3O4 nanocomposites with core–shell or yolk–shell structure, including Fe3O4@SnO2,11 Fe3O4@TiO2,12 Fe3O4@ZnO,13 have been reported. However, as an important microwave absorber, Fe3O4 particles show some shortcomings: such as narrow absorption frequency, high filler content, poor environmental stability; while, narrow absorption frequency, the high density and poor environmental stability of these nanocomposites will restrict the wide application as excellent microwave absorber which should exhibit the advantages of strong absorption, wide absorption frequency, lightweight, and high stability.14,15 Furthermore, coating magnetic nanomaterials with an insulting material is realized as an effective way to increase the surface anisotropic energy and prevent the core materials from oxidation. In the case of silica-coated iron oxide, the coating materials can also disperse on the surface more uniformly owing to the existence of hydroxyl groups, in addition, a better match of the dielectric loss and magnetic loss may be realized by the existence of the protective silica shell.16
At the meantime, compared with traditional microwave absorption materials, reduced graphene oxide (RGO) has been applied as a new wave absorption material because of its desirable physical and chemical properties.17,18 Recently, Wang et al. reported a series of materials coated by RGO sheets which showed enhanced microwave absorption.6,19–21 Generally, the enhanced microwave absorption of these RGO-based materials may mainly arise from large aspect ratio, high conductivity and residual defects on the surface of the RGO sheets,22,23 which cause electronic dipole polarization and interfacial polarization.
In this paper, we introduce the synthesized Fe3O4 magnetic nanoparticle clusters with relatively uniform sizes by hydrothermal reaction. The chemical stability of magnetic nanoparticle clusters was improved by silica coating. Moreover, Fe3O4@SiO2@RGO nanocomposites were synthesized under ultrasonic treatment, and their wave absorption properties and the mechanism of enhanced wave absorption properties were also studied.
Experimental section
Materials
Ferric chloride hexahydrate (FeCl3·6H2O), anhydrous sodium acetate, ethylene glycol (EG), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), tetraethyl orthosilicate (TEOS, 98 wt%), ammonia solution (28 wt%), and anhydrous ethanol were all purchased from Alfa Aesar. All chemicals were of analytical reagent grade and were used as received.Preparation of Fe3O4 magnetic nanoparticle clusters (MNCs)
In the synthesis process of about 200 nm MNCs, FeCl3·6H2O (2.028 g), NaAc (3.69 g), and EDTA-2Na (0.023 g) were dissolved in ethylene glycol (60 mL) under stirring. Then ultrasonic treatment for 20 min, the obtained homogeneous dark yellow solution was transferred to a Teflon-lined stainless-steel autoclave and heated at 200 °C for 10 h and then naturally cooled down to room temperature. After that, the dark precipitates were washed with water and ethanol three times each other, and then dried in vacuum for 24 h.Preparation of Fe3O4@SiO2 microspheres
For silica coating, the core–shell Fe3O4@SiO2 microspheres were synthesized through a modified Stöber method. Typically, 0.0258 g of the as-prepared Fe3O4 MNCs were dispersed in a mixture of ethanol (40 mL), water (6 mL), and ammonia solution (1 mL). Briefly, TEOS (0.17 mL) was added into the above dispersion and the reaction was allowed to proceed for 1 h under stirring and ultrasonic treatment. Then the product was collected with the help of magnet and washed three times with ethanol and water, and dried at 60 °C for 8 h under vacuum.Preparation of Fe3O4@SiO2@RGO nanocomposites
Typically, graphite oxide (GO) was synthesized by a modified Hummers method.24 The RGO synthesis process was the same with Wang and his co-workers previously reported.25 To get the nanocomposites, 30 mg of Fe3O4@SiO2 microspheres was added to RGO suspension and sonicated for 2 h. Afterwards, the product was isolated by centrifugation and washed with ethanol, and finally freeze-dried.Characterization
The samples XRD data were measured using X-ray diffractometer (D/MAX-1200, Rigaku Denki Co. Ltd, Japan) with Cu Ka radiation at λ = 0.15406 nm. To observe the morphology, size and microstructure of the nanocomposites, scanning electron microscopy (SEM, JSM-7500F) and transmission electron microscopy (TEM, JEOL-2100F) were used. The lakeshore vibrating sample magnetometer (VSM, Riken Denshi Co. Ltd, Japan) was used to measure the magnetic properties of the samples at room temperature.Microwave absorption performance measurement
The composites used for EM absorption measurement were prepared by mixing the products with paraffin wax in different mass percentages and pressed into a cylindrical shaped compact (Φout = 7.00 mm and Φin = 3.04 mm). The EM parameters of the composites were measured by the transmission/reflection coaxial line method. And the values of complex permittivity and permeability were measured in the 2–18 GHz frequency range by an Agilent N5244a net work analyzer.Results and discussion
As shown in Fig. 1a, the Fe3O4 MNCs display monodispersed characteristic with the diameter in the range of 180–220 nm. It can be observed that the as-prepared MNCs with rough surface suggested such secondary structure was built up by subunit grains. Fig. 1b shows a high-resolution TEM image of the individual Fe3O4 MNC. It can be clearly seen that regularly paralleled lattice fringes with the space between neighboring lattices is about 0.29 nm, which indicates the single-crystal nature of the specific subunit nanocrystals.26 From Fig. 1c, it indicates that the core–shell structure of the Fe3O4@SiO2 microspheres. It is visible that the smooth surface and a uniform SiO2 layer encapsulates the Fe3O4 MNC. And the inset of figures indicate the thickness of SiO2 is about 30 nm. Moreover, Fig. 1d provides a representative image of the Fe3O4@SiO2@RGO nanocomposites, and the Fig. S1† shows the FESEM image of the Fe3O4@SiO2@RGO nanocomposites and corresponding elemental maps of Fe, Si, O and C, it can confirm that the Fe3O4@SiO2 nanoparticles were coated by RGO absolutely.To confirm the phases and structures of the as-prepared samples, the XRD of Fe3O4, Fe3O4@SiO2, and Fe3O4@SiO2@RGO were shown in the Fig. 2a. From the XRD pattern of Fe3O4 MNCs, it can be observed that all the diffraction peaks are readily indexed to the face-centered cubic structure of Fe3O4 (JCPDS no. 88-0315). The narrow sharp peaks mean that the Fe3O4 magnetic nanoparticle clusters are highly crystallized. Moreover, the lower XRD pattern of Fe3O4@SiO2 is almost same as that Fe3O4 MNCs. Therefore, no peaks from SiO2 can be confirmed implying that formed SiO2 shell is amorphous phase. In addition, the absence of X-ray diffraction sharp peak for graphite at around 26° suggests that the RGO nanosheets disperse on the surface of Fe3O4@SiO2 microspheres homogeneously.27,28 The electronic structure of graphite and graphene-based materials can be analysed by Raman spectroscopy. Fig. 2b represents the Raman spectra of GO and Fe3O4@SiO2@RGO nanocomposites. It can be observed that there are no obvious changes of the positions and shapes of the D band and G band of RGO in the nanocomposites, compared with GO. The intensity ratio of ID/IG is used to evaluate the ordered and disordered crystal structures of carbon.29 The ID/IG ratio is 1.14 for GO, and 1.43 for the composites. The change in ID/IG ratio explains the fact that the reduction of GO leads to smaller but more numerous sp2 domains in the carbon.30
 | ||
| Fig. 2 (a) XRD patterns of Fe3O4 magnetic nanoparticle clusters, Fe3O4@SiO2 microspheres and Fe3O4@SiO2@RGO nanocomposites; (b) Raman spectra of GO and Fe3O4@SiO2@RGO. | ||
The magnetization of the as-prepared various samples were measured at room temperature, and presented in Fig. 3. The saturation magnetization (Ms) and coercivity (Hc) values were shown at Table 1. The Ms of the Fe3O4@SiO2 and Fe3O4@SiO2@RGO decrease due to the presence of the SiO2 and RGO coating, however, the Hc values instead increase suggesting the larger anisotropic energy. In addition, these three kinds of samples express typical ferromagnetic behavior.
 | ||
| Fig. 3 Magnetization hysteresis loops of the Fe3O4 MNCs, Fe3O4@SiO2 microspheres, and Fe3O4@SiO2@RGO nanocomposites measured at room temperature. | ||
| Sample | Ms (emu g−1) | Hc (Oe) |
|---|---|---|
| Fe3O4 | 70.3 | 30.2 |
| Fe3O4@SiO2 | 25.1 | 45.1 |
| Fe3O4@SiO2@RGO | 17.1 | 55.6 |
Owing to the special structure, the samples may have good microwave absorption properties. Therefore, to test microwave absorption performance, various contents of samples mixed with paraffin wax to synthesize composites in a press process. Based on the measured data of permittivity and permeability, the reflection loss (RL) values can be calculated by the following expression:31,32
 | (1) |
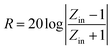 | (2) |
It can be observed that the Fe3O4@SiO2@RGO + wax composites exhibit enhanced wave absorption properties. Fig. 4a shows the theoretical reflection loss (RLs) of composites 20 wt% Fe3O4, 10 wt% Fe3O4@SiO2, 20 wt% Fe3O4@SiO2, 10 wt% Fe3O4@SiO2@RGO and 20 wt% Fe3O4@SiO2@RGO, with paraffin wax at a thickness of 3.0 mm. It shows that the RLs of Fe3O4@SiO2@RGO + wax composites are much higher than other composites, especially at a filler content of only 20 wt%. The minimum reflection loss of the composites reaches −26.6 dB at 9.7 GHz, and the frequency bandwidth less than −10 dB is from 8.4 to 11.8 GHz. Moreover, at the filler content of 10 wt%, the RLs can reach 15.4 dB at 11.4 GHz, and the frequency bandwidth less than −10 dB is from 11.1 to 14.1 GHz. Fig. 4b shows the calculated theoretical RLs of Fe3O4@SiO2@RGO + wax composites at various thicknesses (2–5 mm) with the filler loading of 20 wt%. It indicates that the microwave absorbing properties and the minimum RLs corresponding to the maximum absorptions gradually appeared in different frequency can be tunable by controlling the thickness of the absorbers. And it worth noted that the materials attained a reflection loss of less than −10 dB in the frequency range from 4.4 to 17.3 GHz with an absorber thickness of 2.0–5.0 mm, confirming that this kind of composite is a broadband wave-absorbing material, which is quite beneficial to many electromagnetic shielding material designed to reduce electromagnetic waves over a wide frequency range.33,34 With the lower filler content, the composites can have strong absorption and wide absorption frequency, compared with other Fe3O4-based materials reported as microwave absorber.9,14 To demonstrate the Fe3O4@SiO2@RGO nanocomposites greatly enhanced the wave absorption properties, for comparison, the three-dimensional presentations of RLs were also shown in Fig. 4c and d, the images displayed the RLs of Fe3O4@SiO2@RGO + wax and Fe3O4@SiO2 + wax with different thicknesses in the range of 2–18 GHz with the same loading. Except for the enhanced wave absorption properties, the Fe3O4@SiO2@RGO + wax materials are still as flexible and can be made into different shapes.
To investigate the possible electromagnetic wave absorption mechanism of samples, the frequency dependence relative permittivity and permeability for materials are displayed in Fig. 5. The real permittivity and permeability are connected with the storage ability of electromagnetic energy, whereas the imaginary permittivity and permeability are linked with energy dissipation and magnetic loss.35 It can be seen that the values of ε′ and ε′′ for Fe3O4@SiO2@RGO + wax composites are much larger than pure Fe3O4 + wax and Fe3O4@SiO2 + wax, which indicates that RGO can greatly improve the dielectric properties of composites. The ε′ values of 20 wt% Fe3O4@SiO2@RGO + wax are in the range of 6.7–9.2, which is slightly larger than 10 wt% Fe3O4@SiO2@RGO + wax, while the ε′′ values vary from 1.9–2.5, suggesting the strong dielectric loss in almost all frequency region. Moreover, the variation tendency of μ′ and μ′′ for these composites are basically the same, especially the negative μ′′ values appear in composites, which signifies the magnetic energy radiate out without any absorption.36
 | ||
| Fig. 5 (a) Real and (b) imaginary parts of relative complex permittivity; (c) real and imaginary parts of relative complex permeability for paraffin wax composites in the frequency range of 2–18 GHz. | ||
It can be concluded that the values of dielectric tangent loss are higher than magnetic tangent loss for Fe3O4@SiO2@RGO composites from Fig. S2,† which means that the main loss mechanism is dielectric loss rather than magnetic loss. The maximum value of dielectric tangent loss is 0.37 (9.7 GHz), which is accordance with the reflection loss peak (shown in Fig. 4a). We need to emphasize that the dielectric loss values are improved dramatically and the magnetic loss values almost have no change after combining with RGO. The causes for dielectric loss include electronic dipole polarization and interfacial polarization.37–39 Firstly, electrons in Fe3O4 can transfer between Fe2+ and Fe3+ ions as EM wave applied, producing dipole polarization in the composites, which has significant effects on the dielectric loss. Moreover, interface polarization arises when the neighboring phases differ from each other in a dielectric constant, conductivity, or both at measuring frequencies. For Fe3O4@SiO2@RGO composites, the interface mainly caused by RGO, so that verifies RGO reported as before is beneficial to EM absorption properties.22,32,40
According to van der Zaag's research achievement,41 for magnetic materials, the magnetic loss mainly derives from eddy current effect, hysteresis, natural resonance, and domain wall resonance.42 However, the hysteresis loss is negligible in the weak field, and the domain wall resonance loss commonly occurs at MHz frequency.43 Therefore, the eddy current effect and natural resonance may be responsible for the attenuation of EM waves over 2–18 GHz frequency range. The eddy current loss can be calculated by the following equation:
| μ′′ ≈ 2πμ0(μ′)2σd2f/3 | (3) |
Another mechanism for magnetic loss is natural resonance, which can be expressed in the following equation:44
| 2πfr = rHa | (4) |
| Ha = 4|K1|/3μ0Ms | (5) |
Conclusions
In summary, the Fe3O4@SiO2@RGO nanocomposites with an obviously enhanced microwave absorption property have been successfully synthesized by combining the versatile sol–gel process with hydrothermal reaction. The nanocomposites (at filler content of only 20 wt%) exhibits excellent microwave absorption properties in terms of both the minimum reflection loss value and the absorption bandwidth over 2–18 GHz. The minimum reflection loss can reach −26.6 dB at 9.7 GHz with the thickness of 3.0 mm, and the absorption bandwidth with a reflection loss below −10 dB up to 12.9 GHz (4.4–17.3 GHz). Therefore, it is believed that the Fe3O4@SiO2@RGO nanocomposites are good candidate for microwave absorption absorbers with strong absorption, wide absorption frequency, lightweight, which are promising for applications in military and commercial fields.Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 51472012), and the Fundamental Research Funds for the Central Universities.Notes and references
- W. Zhu, L. Wang, R. Zhao, J. Ren, G. Lu and Y. Wang, Nanoscale, 2011, 3, 2862–2864 RSC.
- J. Xu, J. Liu, R. Che, C. Liang, M. Cao, Y. Li and Z. Liu, Nanoscale, 2014, 6, 5782–5790 RSC.
- X.-M. Meng, X.-J. Zhang, C. Lu, Y.-F. Pan and G.-S. Wang, J. Mater. Chem. A, 2014, 2, 18725–18730 CAS.
- S. Tyagi, H. B. Baskey, R. C. Agarwala, V. Agarwala and T. C. Shami, Ceram. Int., 2011, 37, 2631–2641 CrossRef CAS PubMed.
- W. Fu, S. Liu, W. Fan, H. Yang, X. Pang, J. Xu and G. Zou, J. Magn. Magn. Mater., 2007, 316, 54–58 CrossRef CAS PubMed.
- X.-J. Zhang, G.-S. Wang, W.-Q. Cao, Y.-Z. Wei, J.-F. Liang, L. Guo and M.-S. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 7471–7478 CAS.
- J. Zou, Z. Wang, M. Yan and H. Bi, J. Phys. D: Appl. Phys., 2014, 47, 275001 CrossRef.
- S. Kolev, A. Yanev and I. Nedkov, Phys. Status Solidi C, 2006, 3, 1308–1315 CrossRef CAS PubMed.
- S. Ni, S. Lin, Q. Pan, F. Yang, K. Huang and D. He, J. Phys. D: Appl. Phys., 2009, 42, 055004 CrossRef.
- J. Wei, J. Liu and S. Li, J. Magn. Magn. Mater., 2007, 312, 414–417 CrossRef CAS PubMed.
- J. Liu, J. Cheng, R. Che, J. Xu, M. Liu and Z. Liu, J. Phys. Chem. C, 2013, 117, 489–495 CAS.
- J. Liu, R. Che, H. Chen, F. Zhang, F. Xia, Q. Wu and M. Wang, Small, 2012, 8, 1214–1221 CrossRef CAS PubMed.
- D. Sun, Q. Zou, Y. Wang, Y. Wang, W. Jiang and F. Li, Nanoscale, 2014, 6, 6557–6562 RSC.
- X. Liu, Y. Chen, X. Cui, M. Zeng, R. Yu and G.-S. Wang, J. Mater. Chem. A, 2015, 3, 12197–12204 CAS.
- X. Li, B. Zhang, C. Ju, X. Han, Y. Du and P. Xu, J. Phys. Chem. C, 2011, 115, 12350–12357 CAS.
- H. Hekmatara, M. Seifi, K. Forooraghi and S. Mirzaee, Phys. Chem. Chem. Phys., 2014, 16, 24069–24075 RSC.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- X.-J. Zhang, G.-S. Wang, Y.-Z. Wei, L. Guo and M.-S. Cao, J. Mater. Chem. A, 2013, 1, 12115–12122 CAS.
- D. Chen, G.-S. Wang, S. He, J. Liu, L. Guo and M.-S. Cao, J. Mater. Chem. A, 2013, 1, 5996–6003 CAS.
- X.-J. Zhang, G.-S. Wang, W.-Q. Cao, Y.-Z. Wei, M.-S. Cao and L. Guo, RSC Adv., 2014, 4, 19594–19601 RSC.
- C. Wang, X. Han, P. Xu, X. Zhang, Y. Du, S. Hu, J. Wang and X. Wang, Appl. Phys. Lett., 2011, 98, 072906 CrossRef PubMed.
- J. Liang, Y. Wang, Y. Huang, Y. Ma, Z. Liu, J. Cai, C. Zhang, H. Gao and Y. Chen, Carbon, 2009, 47, 922–925 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- G.-S. Wang, Y. Wu, Y.-Z. Wei, X.-J. Zhang, Y. Li, L.-D. Li, B. Wen, P.-G. Yin, L. Guo and M.-S. Cao, ChemPlusChem, 2014, 79, 375–381 CrossRef CAS PubMed.
- M. Lin, H. Huang, Z. Liu, Y. Liu, J. Ge and Y. Fang, Langmuir, 2013, 29, 15433–15441 CrossRef CAS PubMed.
- X. Bai, Y. Zhai and Y. Zhang, J. Phys. Chem. C, 2011, 115, 11673–11677 CAS.
- Y. Wang, R. Cheng, Z. Wen and L. Zhao, Eur. J. Inorg. Chem., 2011, 19, 2942–2947 CrossRef PubMed.
- M. Zhang, M. Jia and Y. Jin, Appl. Surf. Sci., 2012, 261, 298–305 CrossRef CAS PubMed.
- J. Su, M. Cao, L. Ren and C. Hu, J. Phys. Chem. C, 2011, 115, 14469–14477 CAS.
- M.-S. Cao, X.-L. Shi, X.-Y. Fang, H.-B. Jin, Z.-L. Hou, W. Zhou and Y.-J. Chen, Appl. Phys. Lett., 2007, 91, 203110 CrossRef PubMed.
- G.-S. Wang, L.-Z. Nie and S.-H. Yu, RSC Adv., 2012, 2, 6216–6221 RSC.
- Y. Zhang, Y. Huang, T. Zhang, H. Chang, P. Xiao, H. Chen, Z. Huang and Y. Chen, Adv. Mater., 2015, 27, 2049–2053 CrossRef CAS PubMed.
- Y. Egami, T. Yamamoto, K. Suzuki, T. Yasuhara, E. Higuchi and H. Inoue, J. Mater. Sci., 2012, 47, 382–390 CrossRef CAS.
- W. Zhou, X. Hu, X. Bai, S. Zhou, C. Sun, J. Yan and P. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 3839–3845 CAS.
- C. Wang, X. Han, X. Zhang, S. Hu, T. Zhang, J. Wang, Y. Du, X. Wang and P. Xu, J. Phys. Chem. C, 2010, 114, 14826–14830 CAS.
- Y. J. Chen, P. Gao, C. L. Zhu, R. X. Wang, L. J. Wang, M. S. Cao and X. Y. Fang, J. Appl. Phys., 2009, 106, 054303 CrossRef PubMed.
- Y.-J. Chen, P. Gao, R.-X. Wang, C.-L. Zhu, L.-J. Wang, M.-S. Cao and H.-B. Jin, J. Phys. Chem. C, 2009, 113, 10061–10064 CAS.
- J. Liu, J. Xu, R. Che, H. Chen, Z. Liu and F. Xia, J. Mater. Chem., 2012, 22, 9277–9284 RSC.
- Y.-Z. Wei, G.-S. Wang, Y. Wu, Y.-H. Yue, J.-T. Wu, C. Lu and L. Guo, J. Mater. Chem. A, 2014, 2, 5516–5524 CAS.
- P. J. van der Zaag, J. Magn. Magn. Mater., 1999, 196, 315–319 CrossRef.
- Y. Du, W. Liu, R. Qiang, Y. Wang, X. Han, J. Ma and P. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 12997–13006 CAS.
- M. Wu, Y. D. Zhang, S. Hui, T. D. Xiao, S. Ge, W. A. Hines, J. I. Budnick and G. W. Taylor, Appl. Phys. Lett., 2002, 80, 4404–4406 CrossRef CAS PubMed.
- C. Kittel, Phys. Rev., 1948, 73, 155–161 CrossRef CAS.
- Y.-J. Chen, G. Xiao, T.-S. Wang, Q.-Y. Ouyang, L.-H. Qi, Y. Ma, P. Gao, C.-L. Zhu, M.-S. Cao and H.-B. Jin, J. Phys. Chem. C, 2011, 115, 13603–13608 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra13315g |
| This journal is © The Royal Society of Chemistry 2015 |



