Antibacterial activities of polymeric poly(dl-lactide-co-glycolide) nanoparticles and Soluplus® micelles against Staphylococcus epidermidis biofilm and their characterization†
Abstract
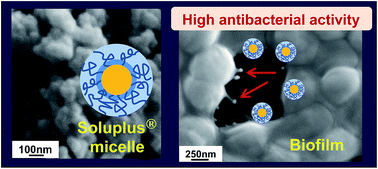
Preparation method of polymeric micelles based on polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer (Soluplus®) have been successfully established as nanocarriers for a drug delivery system on a biofilm formed by Staphylococcus epidermidis. The biodegradable poly(DL-lactide-co-glycolide) (PLGA) nanoparticles were also prepared for comparison. To design effective nanocarriers against biofilm infections, we tested the four types of polymeric nanocarriers on the biofilm: unmodified PLGA, PLGA modified with chitosan (CS), unmodified Soluplus® micelles (Sol), and Sol modified with CS. The antibacterial activity of each nanocarrier against the biofilm was evaluated using field emission scanning electron microscopy (FE-SEM) with hydrophilic ionic liquid (IL) and confocal laser scanning microscopy. FE-SEM results revealed the exact morphology of carriers and bacterial cells at the nanoscale level by an optimized method using IL. The nanocarriers adhering to the biofilm were quantified using the phenol–sulfuric acid method. Antibacterial activity of nanocarriers was determined by minimum inhibitory concentration and minimum bactericidal concentration, and antibacterial assay using LIVE/DEAD BacLight bacterial viability kit. The highest antibacterial activity against the biofilm was demonstrated by a prepared Sol modified with CS. This nanocarrier would be useful for curing biofilm infections. In addition, visualization of the antibacterial activity at the nanoscale level could help in designing effective nanocarriers for target agents.

 Please wait while we load your content...
Please wait while we load your content...