Copper-mediated reaction of oxazirconacyclopentenes with dichlorophenylphosphine: a new pathway for the formation of 1,2-oxaphosphole derivatives†
Yiqing Zhouab,
Sheng Wanga,
Chao Chena and
Chanjuan Xi*a
aKey Laboratory of Bioorganic Phosphorus Chemistry & Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: cjxi@tsinghua.edu.cn
bBeijing National Day School, Beijing 100039, China
First published on 17th August 2015
Abstract
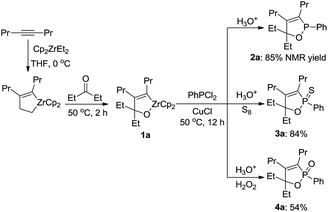
Copper-mediated reaction of oxazirconacyclopentenes with dichlorophenylphosphine afforded 2,5-dihydro-1,2-oxaphosphole in high yields, in which the reaction was performed conveniently in one-pot from an alkyne, carbonyl compound and dichlorophosphine.
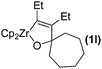
Organophosphorus compounds are important intermediates in organic synthesis and have been widely used as pharmaceutical1 and chemical agents.2 Recently, phosphorus heterocycles3 have received considerable interest because of their unique biological activities4 and wide-ranging utilities as synthetic intermediates in organic synthesis.5 Consequently, much attention has been directed to the synthesis of these compounds. Among them, particular interest was paid to the oxaphosphole derivatives. Although some progress has been achieved for synthesis of the oxaphospholes,6 the most efficient one seems the direct transformation of oxametallacyclopentenes to oxaphospholes. In this regard especially attractive one is oxazirconacyclopentenes, which are easily prepared by the coupling reaction of alkynes and carbonyl compounds with zirconocene–ethylene species.7 The oxazirconacyclopentenes are useful intermediates in a number of organic reactions,7g,8 such as reaction of the oxazirconacyclopentenes with propynoates to afford 2,5-dihydrofurans,8a and reaction with but-2-ynedioates to afford α-methylene-δ-lactone.8b As part of our ongoing project on the chemistry of organozirconium and organophosphorus,9 we herein describe a new pathway for the synthesis of 1,2-oxaphospholes based on the reaction of oxazirconacyclopentenes with dichlorophenylphosphine in the presence of CuCl (eqn (1)).
 | (1) |
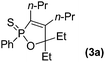
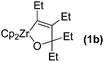
Typical procedure is as follows. To a solution of oxazirconacylopentene 1a in THF (3 mL), prepared from Cp2ZrCl2 (292 mg, 0.6 mmol), EtMgCl (2.0 M THF solution, 0.6 mL, 1.2 mmol), 4-octyne (78 μL, 0.5 mmol), and 3-pentanone (60 μL, 0.5 mmol) according to the reported procedure,7a was added CuCl (100 mg, 1 mmol) and dichlorophenylphosphine (82 μL, 0.6 mmol). The reaction mixture was stirred at 50 °C for 12 h. The reaction mixture was quenched with 3 M HCl and 1,2-oxaphosphole 2a was obtained in 85% NMR yield. Direct purification of 2a by column chromatography on Al2O3 led to the formation of complex inseparable mixture. Since 2a was sensitive under air ambient for direct isolation, this reaction mixture with crude product was treated by addition of elemental sulfur to afford 1,2-oxaphosphole 2-sulfide 3a in 84% isolated yield (Scheme 1). In addition, the reaction mixture was treated with hydrogen peroxide and 1,2-oxaphosphole oxide 4a was isolated in 54% yield. It is notable that the reaction of oxazirconacylopentene with dichlorophosphine did not proceed in the absence of CuCl.
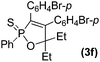
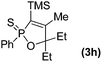
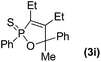
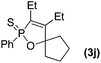
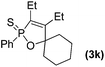
Further study involving the using of various substituted oxazirconacylopentene bearing alkyl, aryl, and TMS groups resulted in all cases in the formation of 1,2-oxaphosphole 2-sulfide in good yields. Some representative examples of the results are summarized in Table 1. Reaction of oxazirconacyclopentenes tolerating four substituents (entries 1–8) with dichlorophenylphosphine gave the corresponding 1,2-oxaphosphole 2-sulfide derivatives in good to high yields. To our delight, the crystals of 3f were suitable for the X-ray diffraction analysis, and its structure was confirmed as 1,2-oxaphosphole 2-sulfide (Fig. 1). When acetophenone was used, trace amount of expected product was observed in GC-MS (entry 9), the starting material remained. It is noteworthy that reaction of spirocyclic oxazirconacyclopentenes with dichlorophenylphosphine gave the corresponding spiro-oxaphospholene 2-sulfide derivatives in high yields (entries 10–12). The expected products, spirooxaphospholene, are popular targets for the development of new biologically active compounds.10
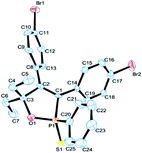 | ||
| Fig. 1 Perspective view of 3f (CCDC 1412383). | ||
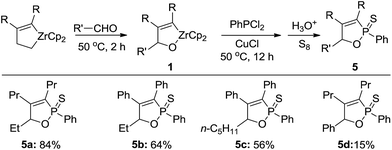
Recently, Liu and co-workers developed an improved procedure for zirconium-mediated alkyne–aldehyde coupling reactions to afford three substituents oxazirconacyclopentenes.7g Reaction of the oxazirconacyclopentenes with dichlorophenylphosphine gave the corresponding 1,2-oxaphosphole 2-sulfide derivatives (Scheme 2). When alkyl aldehyde such as propionaldehyde and hexanal were employed and compound 5a, 5b, and 5c were formed in 84%, 64%, and 56% yield, respectively. It is noteworthy that in the reaction to obtain 5b and 5c, two diastereomers of product 5b and 5c were observed in 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, respectively. When benzaldehyde was used and the product 5d was formed in 15% yield, which could not be fully separated from unidentified by product. Together with result in entry 9 of Table 1, the aryl group on the carbon connected with oxygen in oxazirconacyclopentene gave the poor yield of desired products. That maybe attributed to bulky group.
1 ratio, respectively. When benzaldehyde was used and the product 5d was formed in 15% yield, which could not be fully separated from unidentified by product. Together with result in entry 9 of Table 1, the aryl group on the carbon connected with oxygen in oxazirconacyclopentene gave the poor yield of desired products. That maybe attributed to bulky group.
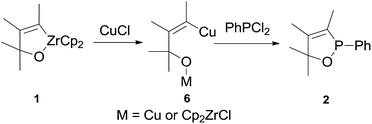
Based on the results obtained here, the following reaction pathway can be proposed for the formation of 2,5-dihydro-1,2-oxaphospholes (Scheme 3). In the first step the Zr–C bond of oxazirconacyclopentene 1 is transmetalated to the Cu–C bond to give 6.11,12 It is not clear whether only the Zr–C bond is transmetalated, or also the Zr–O bond. The intermediate 6 then reacts with dichlorophosphine to give 2,5-dihydro-1,2-oxaphosphole 2.
We conclude that the reaction of oxazirconacyclopentenes with dichlorophenylphosphine in the presence of CuCl gives oxaphosphole derivatives. This reaction represents a convenient pathway to substituted 2,5-dihydro-1,2-oxaphosphole in one-pot from an alkyne, carboxyl compound and dichlorophosphine with zirconocene compound.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21272132, 21472106 and 21032004) and national basic research program of China (2012CB933402).Notes and references
-
(a) J. Collard and C. Benezra, Tetrahedron Lett., 1982, 23, 3725 CrossRef CAS
; (b) I. Morita, K. Kunimoto, M. Tsuda, S. I. Tada, M. Kise and K. Kimura, Chem. Pharm. Bull., 1987, 35, 4144 CrossRef CAS
; (c) Y. Segall, R. L. Grendell, R. F. Toia and J. Casida, J. Agric. Food Chem., 1991, 39, 380 CrossRef CAS
; (d) J. D. Stewart, L. J. Liotta and S. J. Benkovic, Acc. Chem. Res., 1993, 26, 396 CrossRef CAS
; (e) J. W. Darrow and D. G. Drueckhammer, J. Org. Chem., 1994, 59, 2976 CrossRef CAS
; (f) M. M. Mader and P. A. Bartlett, Chem. Rev., 1997, 97, 1281 CrossRef CAS PubMed
; (g) H. Seto and T. Kuzuyama, Nat. Prod. Rep., 1999, 16, 589 RSC
.
-
(a) A. K. Bhattacharya and G. Thyagarajan, Chem. Rev., 1981, 81, 415 CrossRef CAS
; (b) B. E. Marianoff and A. B. Reitz, Chem. Rev., 1989, 89, 863 CrossRef
; (c) J. Motoyoshiya, Trends Org. Chem., 1998, 7, 63 CAS
; (d) N. Kann and T. Rein, Synthesis, 2003, 579 Search PubMed
.
-
(a) N. Bricklebank, Organophosphorus Chem., 2003, 33, 289 CAS
; (b) L. D. Quin, The heterocyclic chemistry of phosphorus: systems based on the phosphorus-carbon bond, Wiley, New York, 1981 Search PubMed
; (c) J. W. Darrow and D. G. Drueckenhammer, J. Org. Chem., 1994, 59, 2976 CrossRef CAS
.
-
(a) I. Morita, K. Kunimoto, M. Tsuda, S. I. Tada, M. Kise and K. Kimura, Chem. Pharm. Bull., 1987, 35, 4144 CrossRef CAS
; (b) S. Racha, C. Vargeese, P. Vemishetti, H. El-Subbagh, E. Abushanab and R. P. Paniza, J. Med. Chem., 1996, 39, 1130 CrossRef CAS PubMed
.
-
(a) G. Keglevich, H. Forintos, G. M. Keserü, L. Hegedüs and L. Tőke, Tetrahedron, 2000, 56, 4823 CrossRef CAS
; (b) V. K. Reddy, J. I. Onogawa, L. N. Rao, T. Oshikawa, M. Takahashi and M. J. Yamashita, Heterocycl. Chem., 2002, 39, 69 CrossRef CAS PubMed
; (c) M. Yamashita, V. K. Reddy, L. N. Rao, B. Haritha, M. Maeda, K. Suzuki, H. Totsuka, M. Takahashi and T. Oshikawa, Tetrahedron Lett., 2003, 44, 2339 CrossRef CAS
; (d) J. Kovács, N. B. Szabó, Z. Nagy, K. Ludányi and G. Keglevich, Heteroat. Chem., 2005, 16, 320 CrossRef PubMed
; (e) T. Novák, J. Deme, K. Ludányi and G. Keglevich, Heteroat. Chem., 2008, 19, 28 CrossRef PubMed
; (f) M. Yamada, M. Yamashita, T. Suyama, J. Yamashita, K. Asai, T. Niimi, N. Ozaki, M. Fujie, K. Maddali, S. Nakamura and K. Ohnishi, Bioorg. Med. Chem. Lett., 2010, 20, 5943 CrossRef CAS PubMed
.
-
(a) M. D. McReinolds, J. M. Dougherty and P. R. Hanson, Chem. Rev., 2004, 104, 2239 CrossRef PubMed
; (b) A. Y. Peng and Y. X. Ding, Org. Lett., 2005, 7, 3299 CrossRef CAS PubMed
; (c) F. Yu, X. Lian and S. Ma, Org. Lett., 2007, 9, 1703 CrossRef CAS PubMed
; (d) F. Yu, X. Lian, J. Zhao, Y. Yu and S. Ma, J. Org. Chem., 2009, 74, 1130 CrossRef CAS PubMed
; (e) P. Fourgeaud, C. Midrier, J. P. Vors, J.-N. Volle, J.-L. Pirat and D. Virieux, Tetrahedron, 2010, 66, 758 CrossRef CAS PubMed
; (f) P. Li, Z.-J. Liu and J.-T. Liu, Tetrahedron, 2010, 66, 9729 CrossRef CAS PubMed
; (g) N. Xin and S. Ma, Eur. J. Org. Chem., 2012, 3806 CrossRef CAS PubMed
; (h) J. H. Hah, B. S. Lee, S. Y. Lee and H.-Y. Lee, Tetrahedron Lett., 2003, 44, 5811 CrossRef CAS
; (i) M. Moura, S. Josse and D. Postel, J. Org. Chem., 2013, 78, 8994 CrossRef CAS PubMed
; (j) A. I. Arkhypchuk, A. Orthaber, V. A. Mihali, A. Ehlers, K. Lammertsma and A. Ott, Chem.–Eur. J., 2013, 19, 13692 CrossRef CAS PubMed
; (k) A. I. Arkhypchuk, M.-P. Santoni and S. Ott, Angew. Chem., Int. Ed., 2012, 51, 7776 CrossRef CAS PubMed
; (l) I. Yavari, A. Alizadeh and M. Anary-Abbasinejad, Tetrahedron Lett., 2003, 44, 2877 CrossRef CAS
.
-
(a) T. Takahashi, M. Kageyama, V. Denisov, R. Hara and E. Negishi, Tetrahedron Lett., 1993, 34, 687 CrossRef CAS
; (b) C. Coperet, E. Negishi, Z. Xi and T. Takahashi, Tetrahedron Lett., 1994, 35, 695 CrossRef CAS
; (c) T. Takahashi, C. Xi, Z. Xi, M. Kageyma, R. Fischer, K. Nakajima and E. Negishi, J. Org. Chem., 1998, 63, 6802 CrossRef CAS PubMed
; (d) T. Takahashi, C. Xi, U. Ura and K. Nakajima, J. Am. Chem. Soc., 2000, 122, 3228 CrossRef CAS
; (e) C. Zhao, T. Yu and Z. Xi, Chem. Commun., 2002, 142 RSC
; (f) C. Zhao, J. Yan and Z. Xi, J. Org. Chem., 2003, 68, 4355 CrossRef CAS PubMed
; (g) S. Guo, H. Zhang, F. Song and Y. Liu, Tetrahedron, 2007, 63, 2009 CrossRef CAS PubMed
.
-
(a) C. Xi, M. Kotora and T. Takahashi, Tetrahedron Lett., 1999, 40, 2375 CrossRef CAS
; (b) Y. Zhou, X. Yan, C. Chen and C. Xi, Organometallics, 2013, 32, 6182 CrossRef CAS
.
-
(a) C. Xi, M. Ma and X. Li, Chem. Commun., 2001, 2554 RSC
; (b) C. Lai, C. Xi, C. Chen, M. Ma and X. Hong, Chem. Commun., 2003, 2736 RSC
; (c) C. Lai, C. Xi, W. Chen and R. Hua, Tetrahedron, 2006, 62, 6295 CrossRef CAS PubMed
; (d) C. Xi, X. Yan and C. Lai, Organometallics, 2007, 26, 1084 CrossRef CAS
; (e) C. Xi, X. Yan, C. Lai, K.-I. Kanno and T. Takahashi, Organometallics, 2008, 27, 3834 CrossRef CAS
; (f) X. Yan and C. Xi, Organometallics, 2008, 27, 152 CrossRef CAS
; (g) X. Yan, B. Yu, L. Wang, N. Tang and C. Xi, Organometallics, 2009, 28, 6827 CrossRef CAS
; (h) X. Yan, C. Lai and C. Xi, Chem. Commun., 2009, 6026 RSC
; (i) Y. Zhou, X. Yan and C. Xi, Tetrahedron Lett., 2010, 51, 6136 CrossRef CAS PubMed
; (j) X. Yan and C. Xi, Acc. Chem. Res., 2015, 48, 935 CrossRef CAS PubMed
.
-
(a) M. J. Camarasa, A. San-Felix, M. J. Perez-Perez, S. Velazquez, R. Alvarez, C. Chamorro, M. L. Jimeno, C. Perez, F. Gago, E. de Clercq and J. J. Balzarini, Carbohydr. Chem., 2000, 19, 451 CrossRef CAS PubMed
; (b) M.-J. Camarasa, A. San-Felix, S. Velazquez, M.-J. Perez-Perez, F. Gago and J. Balzarini, Curr. Top. Med. Chem., 2004, 4, 945 CrossRef CAS
.
-
(a) T. Takahashi, M. Kotora, K. Kasai, N. Suzuki and K. Nakajima, Organometallics, 1994, 13, 4184 Search PubMed
; (b) T. Takahashi, R. Hara, Y. Nishihara and M. Kotora, J. Am. Chem. Soc., 1996, 118, 5154 CrossRef CAS
; (c) T. Takahashi, Z. Xi, A. Yamazaki, Y. Liu, K. Nakajima and M. Kotora, J. Am. Chem. Soc., 1998, 120, 1672 CrossRef CAS
; (d) T. Takahashi, W.-H. Sun, Y. Liu, K. Nakajima and M. Kotora, Organometallics, 1998, 17, 3841 CrossRef CAS
; (e) M. Kotora, C. Xi and T. Takahashi, Tetrahedron Lett., 1998, 39, 4321 CrossRef CAS
; (f) C. Xi, M. Kotora, K. Nakajima and T. Takahashi, J. Org. Chem., 2000, 65, 945 CrossRef CAS PubMed
; (g) C. Chen, C. Xi, Y. Jiang and X. Hong, J. Am. Chem. Soc., 2005, 127, 8024 CrossRef CAS PubMed
; (h) Y. Nishihara, M. Miyasaka, M. Okamoto, H. Takahashi, E. Inoue, K. Tanemura and K. Takagi, J. Am. Chem. Soc., 2007, 129, 12634 CrossRef CAS PubMed
; (i) Y. Nishihara, Y. Okada, J. Jiao, M. Suetsugu, M. Lan, M. Kinoshita, M. Iwasaki and K. Takagi, Angew. Chem., Int. Ed., 2011, 50, 8660 CrossRef CAS PubMed
.
-
(a) M. Ogasawara, S. Arae, S. Watanabe, V. Subbarayan, H. Sato and T. Takahashi, Organometallics, 2013, 32, 4997 CrossRef CAS
; (b) T. Miyaji, Z. Xi, M. Ogasawara, K. Nakajima and T. Takahashi, J. Org. Chem., 2007, 72, 8737 CrossRef CAS PubMed
; (c) S. Doherty, E. G. Robins, M. Nieuwenhuyzen, J. G. Knight, P. A. Champkin and W. Clegg, Organometallics, 2002, 21, 1383 CrossRef CAS
; (d) T. Miyaji, Z. Xi, K. Nakajima and T. Takahashi, Organometallics, 2001, 20, 2859 CrossRef CAS
; (e) S. Doherty, J. G. Knight, E. G. Robins, T. H. Scanlan, P. A. Champkin and W. Clegg, J. Am. Chem. Soc., 2001, 123, 5110 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR data and spectra of compounds for 3a–3h, 3j–3l, 4a and 5a–5c. CCDC 1412383. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra13818c |
| This journal is © The Royal Society of Chemistry 2015 |