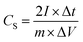Preparation of hierarchically porous carbon nanofoams for electrode materials of supercapacitors†
Yong Zhang,
Youfu Wang and
Aiguo Hu *
*
Shanghai Key Laboratory of Advanced Polymeric Materials, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai, 200237, China. E-mail: hagmhsn@ecust.edu.cn; Fax: +86-21-64253037; Tel: +86-21-64253037
First published on 7th August 2015
Abstract
Hierarchically porous carbon nanofoams (CNFs) with uniform cavity and highly porous skeleton have been prepared via the formation of core–shell organosilica nanoparticles in one-pot reaction and subsequent Friedel–Crafts chemistry. The porosity of the CNFs, which have macroporous cavity (∼50 nm), mesoporous windows (∼12 nm) between adjacent cavities and mesoporous skeleton (∼3 nm), were facially tuned by varying the amount of phenyltriethoxysilane (PTES) used in the first step. CNFs with high surface area (>1100 m2 g−1), large pore volume (∼2 cm3 g−1) and partially graphitized skeleton exhibited good performances as supercapacitor electrode materials. The specific capacitance of the porous CNFs reached 170 F g−1 at a current density of 0.5 A g−1 in an aqueous electrolyte.
Introduction
Supercapacitors are emerging devices for energy storage with higher power density and longer cycling life relative to most batteries. It has become the first candidate for many applications such as electric hybrid vehicles and uninterruptible power supplies. It is the key to develop high performance electrode materials for highly-powered supercapacitors. Porous carbons are widely used materials in supercapacitors because of their low cost, high specific surface area, high electrical conductivity, high stability and tunable pore structures.1–4As demonstrated in many studies, hierarchically porous structure greatly improves the electrochemical performance of the carbon-based electrode materials by providing high electrochemically accessible surface area to the electrolyte and short diffusion pathways.5–16 In general, the hollow macro-cavity plays a role of an ion reservoir ensuring that ion transporting at high rates; the interconnected mesopores provide low-resistant pathways for the ions to the inner-pore surface. The micropores maximize the space for ion storage and charge transfer reactions and hence enhance the capacitance.17,18
Recently, a kind of 3D hierarchically porous carbons (HPCs) with interconnected structure has been reported that could improve the rate capacitance of supercapacitors relative to general porous carbon materials, where energy and power limitations were normally observed at high rates for the resistance and the tortuous diffusion pathways.17 Cheng et al.17 designed a 3D aperiodic hierarchically porous graphitic carbon (HPGC) with macroporous cores, mesoporous walls, and micropores by using hard template method, and they first demonstrated the formation of macroporous ion-buffering reservoirs and graphitic mesoporous walls. The 3D HPGCs showed both high energy and power densities at high rates. The excellent performance of the HPGCs at high rates clearly confirms that the 3D hierarchically porous structure facilitates ion transport and keeps a high accessible surface area for energy storage. Liang et al.16 synthesized hierarchically porous electrode materials possessing higher energy and power density than ever reported, which can be used to replace batteries for energy storage. It was proved that mesoporous mass transport pathway and macroporous ion-buffer reservoirs are very useful in improving ion diffusion properties in HPCs, and further investigation revealed that the synergetic effect of the macropores and mesopores imparted the porous carbon with good ion accessibility and fast ion transport.19 At present, there still constitute challenges for the applications of HPCs. HPCs are mainly prepared through complicated and tedious template-incorporated methods, while the template-free methods might show a relatively poor control of the pore structure. Thus, simple, effective, and controllable methods are still highly desired.
Herein, we described a simple and facile method to prepare CNFs through the synthesis of core–shell organosilica nanoparticles in one-pot reaction and subsequent Friedel–Crafts chemistry.20 The synthesized CNFs showed hierarchically porous structures with high surface area, large pore volume and partially graphitized skeleton, exhibiting good performances as supercapacitor electrode materials. The specific capacitance of the porous CNFs reached 170 F g−1 at a current density of 0.5 A g−1 in an aqueous electrolyte.
Experimental section
Materials
Tetraethyl orthosilicate (TEOS), phenyltriethoxysilane (PTES), FeCl3 (anhydrous) and polytetrafluoroethylene (PTFE) were obtained from Aladdin Reagent Inc. 1,2-Dichloroethane (DCE) was obtained from National Medicines Corporation Ltd. of China. Formaldehyde dimethyl acetal (FDA) was obtained from Alfa Aesar Co. Other chemicals used in the reactions were commercially available and used without further purification. All of these chemicals were of analytical grade. Distilled water (DW) was used in all the experiments.Synthesis of hybrid SNPs via one-pot method
Core–shell hybrid silica nanoparticles (SNPs) with different shell hybrid degrees were prepared via one-pot method following previous reports21 with minor modification. Hybrid SNPs with the diameter of ∼50 nm was prepared at 50 °C. TEOS (6.0 mL) was added into a mixture of ammonium hydroxide solution (25 wt%, 7.5 mL), DW (2.5 mL) and ethanol (150 mL). After stirring for 2 h, a mixture of TEOS (3 mL) and PTES (1 mL) was added and allowed to react for another 3 h. Then, the system was evaporated to remove ethanol and ammonia and dried under vacuum at 80 °C to obtain white powders. The final hybrid SNPs was denoted as HSNP-A, HSNP-B and HSNP-C, according to the amount of PTES as 1.0 mL, 1.5 mL and 2.0 mL, respectively.Cross-linking the phenyl groups on the hybrid SNPs
Cross-linking the phenyl groups on the shell of the hybrid SNPs was processed following the previous reports22,23 with Friedel–Crafts chemistry. For HSNP-A, FDA (20.7 mmol) was added to DCE (120 mL) containing SNP@Ph-SiO2 (2.96 g) and FeCl3 (20.7 mmol). After degassing by N2 bubbling, the mixture was stirred at 45 °C for 5 h to form preliminary polymeric network, and then heated at 80 °C for 19 h to complete the cross-linking. After cooling to room temperature, the obtained cross-linked hybrid SNPs was filtered and washed with a mixture of methanol and HCl (v/v = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for three times and further dried under vacuum at 80 °C to obtain yellow brown powder.
1) for three times and further dried under vacuum at 80 °C to obtain yellow brown powder.
Carbonization
Carbonization of the cross-linked hybrid SNPs was performed in a tube furnace with a continuous mixed gas flow (H2/Ar 10%). Cross-linked hybrid SNPs were heated in the furnace from room temperature to 500 °C at a heating rate of 5 °C min−1 and kept for 2 h and then to 900 °C and maintained for 1 h. After cooling to room temperature, a black powder was obtained.Removal of the silica template
CNFs were obtained after removing the silica from carbonized hybrid materials with HF solution (40 wt%). The removal of silica template was performed in polytetrafluoroethylene (PTFE) beaker with good ventilation. After washing with DW and drying under vacuum, CNFs were obtained as black powders. The obtained CNFs were denoted as CNF-A, CNF-B and CNF-C, where A, B and C have the same meaning as stated above.Material characterization
The sizes and morphologies of the samples were determined by transmission electron microscopy (TEM, JEOL JEM1400, 100 kV). Raman measurements were performed on an in Via-reflex Raman spectrometer (Renishaw, 514 nm). Nitrogen sorption isotherms were measured at 77 K with an ASIQM0000-4 (Quantachrome) instrument. Before measurements, the samples were degassed under vacuum at 200 °C for at least 6 h. The surface area of the samples was determined from the Brunauer–Emmett–Teller (BET) equation and analysed with Barrett–Joyner–Halenda (BJH) method, and the pore volume and pore size distributions were derived from the desorption branches of the isotherms.Electrochemical tests
All the electrochemical measurements were processed using a two-electrode setup with a titanium mesh coated with the CNFs serving as the working electrodes. For the preparation of the working electrodes, 85 wt% of the CNF powder, 10 wt% of carbon black and 5 wt% of PTFE were mixed with a few drops of ethanol. After grinding for 1 h, homogeneous black slurry was obtained. Then, the slurry was tailored to a little circle slice (1–2 mg) and pressed onto titanium mesh current collector (1 cm × 1 cm) and dried at 100 °C for 12 h to fabricate the electrode. Electrochemical performance was tested by cyclic voltammetry (CV), galvanostatic charge/discharge (CD) and electrochemical impedance spectroscopy (EIS) on a CHI 660D electrochemical workstation using two-electrode sandwich-type cell supercapacitors at room temperature. The cell supercapacitors were composed of two symmetrical working electrodes sandwiched by a separator and the aqueous electrolyte solution of 6 M KOH. The potential range for CV and CD experiments was 0–0.8 V. EIS test was carried out in the frequency range of 105 to 10−2 Hz with 5 mV amplitude corresponding to open circuit potential. The cycling stability was tested using two electrode cell supercapacitors on a LAND CT2001A program testing system.The specific capacitances (CS, F g−1) of the electrode materials were calculated from the CD curves according to the following equation:
Results and discussion
Scheme 1 illustrates the preparation of CNFs from hybrid SNPs consisting of four steps: (a) synthesis of core–shell hybrid SNPs via one-pot method, (b) cross-linking the phenyl on the shell of the hybrid SNPs, (c) carbonization and (d) removal of silica template. The core SNPs were prepared by the well-known Stöber method. Then in situ addition of a mixture of PTES and TEOS with different ratios reached the different hybrid degrees (volume ratio of PTES/TEOS) of the shell. When the hybrid degree was too low, the final CNFs could not self-support after the template removal, while when the hybrid degree was too high, amorphous carbon appeared probably due to the self-condensation of the PTES in the second stage. | ||
| Scheme 1 Schematic illustration of the synthesis of hierarchically porous carbon nanofoams (CNFs) through cross-linking and carbonization of hybrid SNPs. | ||
The size and the morphology of the hybrid SNPs were observed with TEM. As shown in Fig. 1A–C, three SNPs exhibited core–shell nanostructure and uniform core size of about 50 nm. With the increase of the hybrid degree, the surface of hybrid SNPs become more and more rough, which is probably due to the formation of less dense structure when more hydrophobic phenyl group is incorporated into the shell.
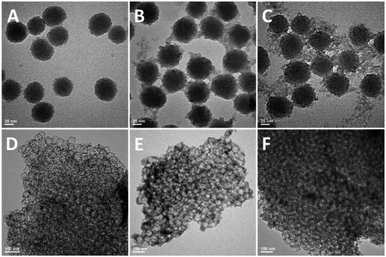 | ||
| Fig. 1 TEM images of hybrid SNPs and CNFs. (A) HSNP-A, (B) HSNP-B, (C) HSNP-C, (D) CNF-A, (E) CNF-B and (F) CNF-C. | ||
The cross-linking of phenyl groups on the shell of hybrid SNPs was performed with a low-cost versatile Friedel–Crafts type “knitting” strategy22,24,25 recently developed by Tan et al. By using FeCl3 as a catalyst, FDA as a cross-linking agent, this reaction generates rigid aromatic network in a facile manner, with the only by-product as methanol. After cross-linking, the hybrid SNPs turned from white to brown yellow, showing the formation of the crosslinked network.
Fig. 1D–F shows the TEM images of the CNFs. Foam-like structures are present in all the samples with different hybrid degrees. When this number reaches 33% (CNF-A), CNFs exhibited integrated and thin foam structure. When this number reaches 50% (CNF-B), structurally ill-defined matter appeared which may originate from the self-condensation of PTES. When this number reaches 66% (CNF-C), the skeleton of CNF-C was much denser with ill-defined structures. All the CNFs show interconnected porous structure, with the hybrid degree increased gradually, the foam skeleton become more and more thick and fuzzy. This might be due to the phase separation induced by the incompatibility of phenyl group and the silica phase and the self-condensation of PTES.
The porosity of CNFs was analysed with nitrogen sorption isotherms (Fig. 2A). All the plots show a representative type-IV curve with a hysteresis in the P/P0 range of 0.4–1.0. This adsorption behaviour is attributed to the capillary condensation of N2 in the mesoporous windows and the skeleton of the CNFs. The pore size distributions of the CNFs were calculated with BJH method from the desorption branches as shown in Fig. 2B. All the CNFs show hierarchically porous structures. Sharp peaks at pore size of 3 nm appear in all the CNFs, which may come from the expansion of the intrinsic micropores of the cross-linked hybrid SNPs during carbonization. CNF-A and CNF-B also exhibit a mesopores of about 12 nm, which may come from the windows between adjacent cavities. However, for HSNP-C, the compact phenyl in the shell may form intact pore wall in CNF-C after cross-linking and carbonization, hindering the formation of the inter-cavity windows. We can also see the porous cavity of 50 nm originating from the silica core with the DFT method (see Fig. S1 in ESI†).
The texture parameters of CNFs are showing in Table 1. It can be seen that the three materials have large specific surface area and pore volume. With the increase of the hybrid degree during the formation of the HSNPs, the total pore volume and surface areas of the CNFs decrease, illustrating the evolution of the porous structure of CNFs. The porous structure of the CNFs is gradually built-up with the improvement and perfection of the phenyl network by the co-condensation of PTES and TEOS at the first step. However, when excess of PTES was added, the amorphous carbon will form due to the self-condensation of PTES, resulting in thick and unconnected walls. The large surface area (1174 m2 g−1) and large pore volume (1.97 cm3 g−1) of CNF-A may result from the formation of rich mesoporous skeleton due to the low but enough hybrid degree to form the carbon skeleton.
| Samples | Surface area (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|
| CNF-A | 1174 | 1.972 |
| CNF-B | 1100 | 1.775 |
| CNF-C | 1038 | 0.945 |
Raman spectroscopy was used to characterize the graphitization degree of the carbon materials. As shown in Fig. 3, all the CNFs show a similar curve, with two peaks at ∼1350 and 1500 cm−1, corresponding to the D and G bands of carbon materials. The D band is due to lattice defects of carbon, while G band is the characteristic peak of the graphitic structure. The CNFs with different hybrid degrees exhibited similar Raman spectra, showing that the graphitization degree is independent of the topological structure, as they went through similar carbonation process. The intensity ratio (ID/IG) of CNFs is close to 0.90, showing a partially graphitized degree in the carbonization process. The partially graphitized walls and the hierarchically interconnected pore structure with large surface area and pore volume make these CNFs potential candidates as electrode materials for supercapacitors.
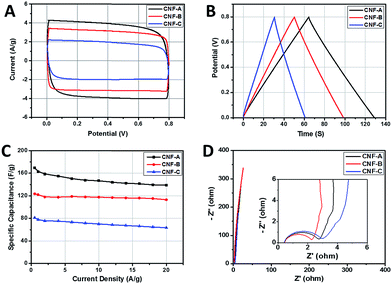
The electrochemical performances of the porous CNFs were evaluated with cyclic voltammetry (CV) and galvanostatic charge/discharge (CD) tests. As shown in Fig. 4A, the CV curves of the CNFs at a high scan rate of 50 mV s−1 are characteristic of a regular rectangle. As the scan rate increase, CV curves (Fig. S2A, S3A and S4A†) for the CNFs electrode material become tilted but still maintain a rectangular-like shape, indicating a fast charge–discharge process in the CNFs. The obvious increase of the current with the scan rate suggests good rate capabilities of these porous electrode materials.
The CD curves (Fig. 4B) of these CNFs at a current density of 1 A g−1 exhibit mirror-like triangular-shaped charge/discharge curves with no obvious voltage drop, indicating typical electrical double-layer capacitance. The CD curves (Fig. S2B, S3B and S4B†) of CNFs under different current densities remain triangle-shaped curves even at high current density (>10 A g−1), which reveals that the CNFs electrodes are suitable for supercapacitors where high loading current charge/discharge is required.
Fig. 4C presents the specific capacitance as a function of the current density of CNFs. With the increase of current density from 0.5 to 20 A g−1, CNF-A exhibits specific capacitance from 170 to 139 F g−1, with capacitance retention of 82%. CNF-B shows specific capacitance from 124 to 113 F g−1, with capacitance retention of 91%. The CNF-C has the lowest specific capacitance from 81 to 63 F g−1, with capacitance retention of 78%. And the performance of CNFs in electronic storage is competitive with recently reported porous carbon materials (Table S1 in ESI†). The larger the accessible surface area of the CNFs, the higher capacitance, agreeing well with the Helmholtz equation.2
Electrochemical impedance spectroscopy (EIS) is a powerful tool to evaluate the conductive properties of electrode materials. The EIS spectra of these porous CNFs are obtained in a frequency range of 100 kHz to 0.01 Hz at open circuit potential with an alternating current perturbation of 5 mV. In the low frequency region, the straight line is attributed to the ion diffusion in the electrolyte. The linear parts (Fig. 4D) of CNFs tend to be vertical lines, indicating the fast ion diffusion and good capacitive retention in the CNFs. At the high frequency end, the intercept at the real axis represents the ohmic resistance (RS), including the resistance of electrolyte solution, intrinsic resistance of substrate and contact resistance at the interface active material/current collector. All the CNFs show the similar RS values due to the hierarchical structure and similar graphitization degrees. The semicircle corresponds to the charge transfer resistance (RCT) at the electrode/electrolyte interface. With the current density increasing, the lower ohmic resistance of CNFs shows less influence on the dynamic accommodation of the hydrated ions at the surface of electrode, resulting in high rate capability. CNF-B exhibited the highest rate capability (Fig. 4C) due to the smallest RCT which is consistent with the structural evolution observed in TEM and pore structure analysis, implying that the hierarchical structure of the CNFs is important to their ability of accommodating the electrolyte ions.
Fig. 5 shows the cycling stability of the CNF-B at a current density of 1 A g−1 in 6 M KOH solution. The capacitance retention of CNF-B are up to 85% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, indicating good cycling stability of the CNFs (Fig. S5 in ESI†) as electrode material. CNF-B shows better cycling stability than CNF-A and CNF-C due to the lower inner resistance as shown in Fig. 4D.
000 cycles, indicating good cycling stability of the CNFs (Fig. S5 in ESI†) as electrode material. CNF-B shows better cycling stability than CNF-A and CNF-C due to the lower inner resistance as shown in Fig. 4D.
 | ||
| Fig. 5 Cycling stability for supercapacitor based on CNF-B measured at current density of 1 A g−1 in a two-electrode system by using 6 M KOH aqueous solution as electrolyte. | ||
Conclusions
In summary, hierarchically porous CNFs with macroporous cavity and mesoporous windows and skeleton were synthesized through Friedel–Crafts cross-linking of phenyl group on the shell of hybrid SNPs and followed by carbonization. The CNFs with large surface area, large pore volume, and partly graphitized skeleton can be used as electrode materials of supercapacitors. It was demonstrated that the hierarchically porous structure of the CNFs favours the rapid diffusion of electrolyte ions and good conductivity, resulting in good capacitive performance, and excellent long-term cycling stability. These CNFs exhibited a maximum specific capacitance of 170 F g−1 in aqueous electrolytes. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles, retention of 85% of the initial capacitance was observed, suggesting the excellent electrochemical and structure stability of CNFs electrode materials.
000 continuous cycles, retention of 85% of the initial capacitance was observed, suggesting the excellent electrochemical and structure stability of CNFs electrode materials.
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (91023008, 21474027), the Fundamental Research Funds for the Central Universities and the Shanghai Leading Academic Discipline Project (B502). A. H. thanks Prof. Gengchao Wang for his help in electrochemical tests in this work.Notes and references
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS PubMed.
- L. L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- Y. P. Zhai, Y. Q. Dou, D. Y. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- W. Xiong, M. X. Liu, L. H. Gan, Y. K. Lv, Z. J. Xu, Z. X. Hao and L. W. Chen, Colloids Surf., A, 2012, 411, 34–39 CrossRef CAS PubMed.
- X. M. Ma, M. X. Liu, L. H. Gan, Y. H. Zhao and L. W. Chen, J. Solid State Electrochem., 2013, 17, 2293–2301 CrossRef CAS.
- Z. Chen, J. Wen, C. Z. Yan, L. Rice, H. Sohn, M. Q. Shen, M. Cai, B. Dunn and Y. F. Lu, Adv. Energy Mater., 2011, 1, 551–556 CrossRef CAS PubMed.
- F. J. Li, M. Morris and K. Y. Chan, J. Mater. Chem., 2011, 21, 8880–8886 RSC.
- M. Rose, Y. Korenblit, E. Kockrick, L. Borchardt, M. Oschatz, S. Kaskel and G. Yushin, Small, 2011, 7, 1108–1117 CrossRef CAS PubMed.
- B. You, J. H. Jiang and S. J. Fan, ACS Appl. Mater. Interfaces, 2014, 6, 15302–15308 CAS.
- Q. L. Zhao, X. Y. Wang, C. Wu, J. Liu, H. Wang, J. Gao, Y. W. Zhang and H. B. Shu, J. Power Sources, 2014, 254, 10–17 CrossRef CAS PubMed.
- Y. Han, X. T. Dong, C. Zhang and S. X. Liu, J. Power Sources, 2012, 211, 92–96 CrossRef CAS PubMed.
- Z. S. Wu, Y. Sun, Y. Z. Tan, S. B. Yang, X. L. Feng and K. Mullen, J. Am. Chem. Soc., 2012, 134, 19532–19535 CrossRef CAS PubMed.
- C. Z. Yuan, B. Gao, L. F. Shen, S. D. Yang, L. Hao, X. J. Lu, F. Zhang, L. J. Zhang and X. G. Zhang, Nanoscale, 2011, 3, 529–545 RSC.
- J. L. Huang, J. Y. Wang, C. W. Wang, H. N. Zhang, C. X. Lu and J. Z. Wang, Chem. Mater., 2015, 27, 2107–2113 CrossRef CAS.
- Y. Liang, F. Liang, H. Zhong, Z. Li, R. Fu and D. Wu, J. Mater. Chem. A, 2013, 1, 7000–7005 CAS.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., Int. Ed., 2008, 47, 373–376 CrossRef CAS PubMed.
- S. Dutta, A. Bhaumik and K. C. W. Wu, Energy Environ. Sci., 2014, 7, 3574–3592 CAS.
- F. Xu, R. J. Cai, Q. C. Zeng, C. Zou, D. C. Wu, F. Li, X. E. Lu, Y. R. Liang and R. W. Fu, J. Mater. Chem., 2011, 21, 1970–1976 RSC.
- S. Xu, Y. Luo and B. Tan, Macromol. Rapid Commun., 2013, 34, 471–484 CrossRef CAS PubMed.
- X. D. Huang, K. Qian, J. Yang, J. Zhang, L. Li, C. Z. Yu and D. Y. Zhao, Adv. Mater., 2012, 24, 4419–4423 CrossRef CAS PubMed.
- B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44, 2410–2414 CrossRef CAS.
- Y. Wang, R. Xiong, L. Dong and A. Hu, J. Mater. Chem. A, 2014, 2, 5212–5217 CAS.
- S. J. Xu, K. P. Song, T. Li and B. Tan, J. Mater. Chem. A, 2015, 3, 1272–1278 CAS.
- B. Y. Li, Z. H. Guan, W. Wang, X. J. Yang, J. L. Hu, B. E. Tan and T. Li, Adv. Mater., 2012, 24, 3390–3395 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: More electrochemical capacitive behaviour of CNFs. See DOI: 10.1039/c5ra13235e |
| This journal is © The Royal Society of Chemistry 2015 |