A fast-response, fluorescent ‘turn-on’ chemosensor for selective detection of Cr3+†
Abstract
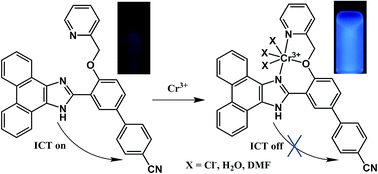
A fast-response, highly selective and sensitive chemosensor, 3, for Cr3+ detection with turn-on fluorescence behavior in the physiological pH range was designed and synthesized. The chemosensor contained a combined push–pull system in which the fluorescent phenanthro[9,10-d]oxazole moiety acts as both an electron donor and a potential binding site. The electron deficient nitrile group served as an electron acceptor. A significant enhancement of fluorescence emission intensities was observed with increasing Cr3+ concentration upon excitation at 300 nm. The emission intensity reached its maximum on adding 8 equiv. of Cr3+ where the quantum yield of 3-Cr3+ was found to be 0.917, ca. 7-fold larger than chemosensor 3. The selectivity mechanism of 3 for Cr3+ was found to be based on the combined effects of the inhibition of ICT and CHEF. Remarkably the entire process was virtually complete in only 10 seconds, with a minimum detection limit for Cr3+ of 1.72 × 10−8 M−1.

 Please wait while we load your content...
Please wait while we load your content...