Low temperature sputtered TiO2 nano sheaths on electrospun PES fibers as high porosity photoactive material†
Abstract
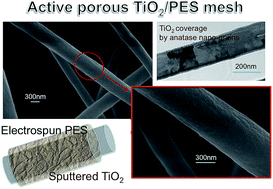
We propose a low temperature approach based on combining an electrospinning methodology and reactive sputtering processes to realise a porous mesh of polyethersulfone (PES) fibers (∼700 nm in diameter) wrapped by TiO2 nano-sheaths, active under UV illumination. The mesh has a porosity as high as ∼91% in volume and was prepared at T ≤ 160 °C. The effectiveness of the TiO2/PES mesh under UV was proved by Methylene Blue (MB) dissociation experiments and related to the TiO2 coverage properties, namely its thickness (∼15 nm), its nano-structuring (grains diameter 8–10 nm) and lattice structure (anatase) that improve the photo-activity action. The combination of electro-spinning plus sputtering processes lays the basis for high porosity and low cost, high throughput, high yield and flexible purifying filter technologies.

 Please wait while we load your content...
Please wait while we load your content...