A molecularly imprinted polymer with flash column chromatography for the selective and continuous extraction of diphenyl amine†
Abstract
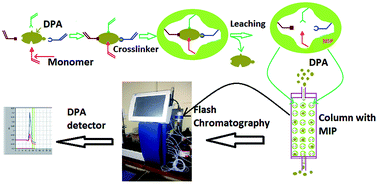
The aim of this study was to prepare MIP for the selective recognition of DPA and its combination with flash column chromatography for the continuous extraction of DPA from ammunition waste. The DPA–MIP was prepared by free radical polymerization using methacrylic acid as a complexing monomer and EGDMA as a crosslinker. Methanol and acetic acid (9 : 1) were used as a leaching agent for DPA. MIP showed maximum adsorption capacity at pH 4. The maximum experimental adsorption capacity obtained for MIP and NIP particles were 31 mg g−1 and 10.2 mg g−1, respectively. Langmuir and Freundlich adsorption isotherm models were used to analyze the experimental data of DPA adsorption on MIP. The Freundlich adsorption isotherm model fit very well with this. Pseudo first order and pseudo second order kinetic models were used to estimate the corresponding rate parameters, equilibrium capacities and correlation coefficients. The selectivity coefficients of MIP for DPA in the presence of NDPA, TNT and tetryl were 23.14, 47.07 and 52.30, respectively. Continuous extraction of DPA was done with the help of flash chromatography combined with MIP and showed more than 98% recovery. MIP has a good regeneration performance and could maintain almost the same adsorption capacity even after three adsorption–desorption cycles.

 Please wait while we load your content...
Please wait while we load your content...