Palladium nanoparticle functionalized graphene nanosheets for Li–O2 batteries: enhanced performance by tailoring the morphology of the discharge product†
Abstract
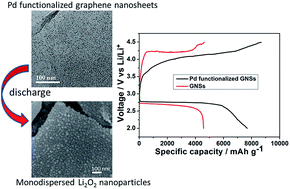
Tailoring the morphology of the cathode discharge product is essential for developing high-capacity and rechargeable lithium oxygen (Li–O2) batteries. In this work, by using functionalized graphene nanosheets (GNSs) with uniformly dispersed palladium nanoparticles (Pd NPs) as the cathode catalyst for the Li–O2 battery, the discharge product of the nano-sized Li2O2 particles formed and homogeneously deposited on GNSs. Through this tailored morphology, the Li–O2 batteries with the Pd functionalized GNS catalyst possessed an increased capacity up to 7690 mA h g−1 at a current density of 0.08 mA cm−2. Meanwhile, reduced charge/discharge overpotentials and good cyclability were obtained.

 Please wait while we load your content...
Please wait while we load your content...