Synthesis, characterization and ion-exchange properties of novel hybrid polymer nanocomposites for selective and effective mercury(ii) removal†
Abstract
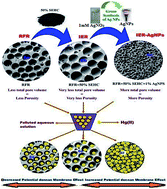
The present work showed the synthesis of Ion Exchange Resin–Silver nanoparticles (IER–AgNPs) by impregnating ecofriendly synthesized AgNPs within a IER and its application as a novel polymer based hybrid nanocomposite for highly efficient removal of mercury (Hg2+) from aqueous solution. The nature of the synthesized IER–AgNPs was structurally and thermally characterized. When compared to resorcinol-formaldehyde resin (RFR) and IER, the synthesized nanocomposites revealed tremendous selectivity for Hg2+ removal from aqueous medium in the non-existence and existence of opposing ions Ca2+, Mg2+ and Na+ at much greater levels than the target poisonous metal. It was found that the potential Donnan membrane effect exerted by the immobilized negatively charged sulfonic acid groups bound to the macroporous cation exchanger of IER result in preconcentration and penetration enhancement of Hg2+ ions prior to their effective segregation by the impregnated AgNPs. Moreover, adsorption isotherms, kinetics, intraparticle diffusion and thermodynamics for the removal of Hg2+ were analyzed. Besides, breakthrough curves were found from column flow studies using the synthetic solution. 10% (w/w) HCl was used as an effective eluting agent for the regeneration of exhausted ion exchangers. Hence, IER–AgNPs cation-exchange resin could be efficiently used for the removal of mercury(II) ions from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...