The effect of the prefrozen process on properties of a chitosan/hydroxyapatite/poly(methyl methacrylate) composite prepared by freeze drying method used for bone tissue engineering
Xueqin Zhang,
Yuxuan Zhang,
Guiping Ma,
Dongzhi Yang and
Jun Nie*
State Key Laboratory of Chemical Resource Engineering, Beijing Laboratory of Biomedical Materials, Beijing University of Chemical Technology, Beijing 100029, PR China. E-mail: niejun@mail.buct.edu.cn; Fax: +86-01064421310; Tel: +86-01064421310
First published on 1st September 2015
Abstract
A chitosan–hydroxyapatite (CS–HA) scaffold reinforced by poly(methyl methacrylate) (PMMA) with good mechanical strength was fabricated. Chitosan–hydroxyapatite scaffolds supplemented with poly(methyl methacrylate) were fabricated using the freeze drying technology and free radical polymerization. Possible applications of the prepared CS–HA/PMMA scaffolds in tissue engineering were tested. The effect of the freeze drying process and chitosan concentration on the properties of the scaffolds were also investigated. The morphology of the CS–HA scaffolds was examined using scanning electron microscopy (SEM). The thermal property of the complex scaffolds was examined using thermogravimetry analysis (TGA). The mechanical property of the bone composites was characterized using an Instron 4505 mechanical tester. Indirect in vitro cytotoxicity tests showed that the bone composite extracts had no significant effect on cell viability. Moreover, in vitro cytocompatibility tests also exhibited a cell population and spreading tendency, which suggest that the CS–HA/PMMA scaffolds were non-toxic to L929 cells. All the results indicated that not only the freeze drying method had significant influence on the properties of the complex scaffolds, but also chitosan concentration had significant influence on the properties of the complex scaffolds. The proposed method could be used to fabricate CS–HA/PMMA bone composites by freeze drying and free radical polymerization, and the fabricated bone composites might have potential applications in the bone tissue engineering scaffold field.
1. Introduction
Annually, numerous people suffer from bone defects caused due to tumours, trauma or bone related diseases and few of them even die because of the lack of an ideal bone tissue.1 During the past decades, autologous and allogenous bone grafts have been used mostly because of their advantages, such as little immune response and good healing.2 However, autologous grafts and allogenous grafts possessed the risk of disease transmission and donor site morbidity.3 The limitations in the use of autologous or allogenous bone grafts has led to the consideration of bone tissue engineering.4The main purpose of bone tissue engineering is to create implantable 3D bone substitutes for skeletal defects and present the current status of translational approaches to engineering bone regeneration.5
Chitosan (CS), a natural cationic polysaccharide, is widely used in combination with polylactic acid or collagen to develop scaffolds for tissue engineering due to its nontoxicity, biofunctionality, biocompatibility and antibacterial nature.6–8 Chitosan-based biomaterials have undergone numerous innovations in the field of tissue engineering and biomedical applications. The valuable aspects of chitosan products, including biocompatibility and biodegradability, have made chitosan an inevitable source for tissue engineering biomaterials.9 Tissue engineering biomaterials should be biocompatible and have appropriate surface chemistry for cell attachment and proliferation. Numerous studies have been focused on the fundamental performance of chitosan, such as molecular weight of components on particles sizes, mechanical properties and network structure of biological tissues, since chitosan is widely used in the biomedical field.10 For example, it is preferable that a tissue engineering construct serves as both a delivery carrier and a three-dimensional (3-D) porous scaffold for cellular activities.11
Hydroxyapatite (HA) is the calcium phosphate mineral found in vertebrate bones, mammalian teeth, fish scales, and the mature teeth of some chitosan species.12 HA is well known due to its excellent bioactivity and biocompatibility and is used in bone tissue engineering currently.13 Furthermore, the osteoconduction, non-inflammation and non-toxicity of HA enable osteoblast adhesion, proliferation and differentiation.14 HA has the unique ability of binding to the natural bone through biochemical bonding, which promotes the interaction between the host bone and grafted material.15,16 It was also demonstrated through in vivo experiments that bone has a greater affinity for implants containing high percentages of HA than those with trace amounts or none.17 However, it is difficult to handle HA and maintain HA in the defect sites because of its brittleness and low plasticity.18
Ice segregation induced self-assembly (ISISA), which is a freeze casting technique, is a green bottom-up method to produce aligned macroporous or layered materials in a sophisticated architecture.19–21 Freezing causes solute or solids in a solution, emulsion, or dispersion to be excluded by an advancing ice front into the interstitial spaces between ice crystals. Subsequent sublimation leads to porous structures.22 By controlling the concentration and freezing direction, complex hierarchical morphologies are produced, which include well-aligned channels, honeycombs, and brick–mortar-bridges. Guiping Ma et al. reported chitosan–sodium hyaluronate polyelectrolyte complex fibres for tissue engineering scaffolds, which were fabricated using the freeze drying technique.23 The applications of freeze drying structures are numerous. Not only can these structures be used in diverse technologies, such as microfluidics and continuous flow catalysts, but also can also be used in tissue regeneration.22 Most studies focused on freezing in a low temperature environment, but there no comparison between directional freezing under liquid nitrogen and freezing under a slowly cooling method.
To overcome the brittleness of hydroxyapatite, chitosan–hydroxyapatite scaffolds can be fabricated using the phase separation and lyophilization technique, rapid prototyping technology, and electrospinning.23 Researchers have carried out numerous studies to overcome the drawbacks of hydroxyapatite such as low mechanical strength, brittleness and low biocompatibility. Chellan Rose et al. reported a collagen chitosan composite scaffold for tissue engineering, which has better mechanical properties.24 Jing Han et al. fabricated a porous PMMA scaffold, which had good mechanical property that matched well with natural bone.25 Zhang et al. prepared biomimetic nanofibers of CS–HA by combining an in situ co-precipitation synthesis approach with the electrospinning process and it was demonstrated that cells could adhere and proliferate well with the biomimetic nanofibers.26 Almeida et al.27 prepared a new poly(methyl methacrylate)-co-ethylhexylacrylate (PMMA-co-EHA) bone cement, which showed excellent biocompatibility.27 Jiang. et al.17 prepared an n-HA/CS scaffold by freeze-drying, which had better cell biocompatibility than the chitosan scaffold in vitro. Ran Kang et al. prepared human induced pluripotent stem cell seeded biomimetic nano-hydroxyapatite (n-HAp) contained polycaprolactone (PCL) nanofibers scaffolds, which were excellent for bone regeneration.28 M. S. Fernández prepared a novel porous multilayered 3D chitosan–hydroxyapatite composite scaffold enriched with fibronectin or extracellular matrix, which could improve the proliferation and differentiation of osteoblast.29
However, the mechanical strength of hydroxyapatite is still not enough for applications. The main purpose of this study is to develop a chitosan–hydroxyapatite scaffold that is reinforced by poly(methyl acrylate) and compare the influence of different freeze drying processes and different chitosan concentrations on the properties of the bone composites and also to develop a potential bone substitute, which has both good biocompatibility and good mechanical properties. As a bone-inducing biopolymer, chitosan is utilized as the framework. HA acts as a high biocompatibility component, which can form a direct bond with the bone and PMMA acts as the load-bearing part. The mechanical properties, and thermal stability of the prepared composites were characterized and the adhesion and proliferation of L929 cells were also measured. It is demonstrated that our scaffolds not only has good mechanical properties, but also good biocompatibility.
2. Experimental section
2.1. Materials
Chitosan (CS, molecular weight of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, about 85% deacetylated) was purchased from Zhejiang Golden-Shell Biochemical Co., Ltd (Zhejiang, China). Hydroxyapatite (HA, particle size of 60 nm, about 96% pure) was purchased from Shanghai Pucheng Biochemical Co., Ltd (Shanghai, China). Methyl methacrylate (MMA, Tianjin Fuchen Chemical Co., Ltd) was purified by distillation under reduced pressure. Benzoyl peroxide (BPO) and N,N-bis(2-hydroxyethyl)-p-toluidine (BHET) were obtained from Xilong Chemical Co., Ltd (Shantou, China) and Fluka Chemical Co., Ltd, respectively. n-Hexane was purchased from Sinopharm Group Chemical Reagent Co. (Beijing, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazole (MTT) was obtained from Alfa Aesar (Massachusetts, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal calf serum (FBS) were purchased from Shanghai Luwen Biochemical Co., Ltd (Shanghai, China). Phosphate buffered saline (PBS) was purchased from Qingdao Haibo Biochemical Co., Ltd (Qingdao, China). Dimethyl sulfoxide and phenol were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Benzoyl peroxide (BPO) was purified by fractional precipitation from a chloroform solution, using methanol as the precipitant.
000 g mol−1, about 85% deacetylated) was purchased from Zhejiang Golden-Shell Biochemical Co., Ltd (Zhejiang, China). Hydroxyapatite (HA, particle size of 60 nm, about 96% pure) was purchased from Shanghai Pucheng Biochemical Co., Ltd (Shanghai, China). Methyl methacrylate (MMA, Tianjin Fuchen Chemical Co., Ltd) was purified by distillation under reduced pressure. Benzoyl peroxide (BPO) and N,N-bis(2-hydroxyethyl)-p-toluidine (BHET) were obtained from Xilong Chemical Co., Ltd (Shantou, China) and Fluka Chemical Co., Ltd, respectively. n-Hexane was purchased from Sinopharm Group Chemical Reagent Co. (Beijing, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide tetrazole (MTT) was obtained from Alfa Aesar (Massachusetts, USA). Dulbecco's modified Eagle's medium (DMEM) and fetal calf serum (FBS) were purchased from Shanghai Luwen Biochemical Co., Ltd (Shanghai, China). Phosphate buffered saline (PBS) was purchased from Qingdao Haibo Biochemical Co., Ltd (Qingdao, China). Dimethyl sulfoxide and phenol were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Benzoyl peroxide (BPO) was purified by fractional precipitation from a chloroform solution, using methanol as the precipitant.
2.2. Methods
| Sample code | Chitosan/g | HA/g | Acetic acid solution/g | Porosity (SCM) | Porosity (RCM) |
|---|---|---|---|---|---|
| CS-3/HA-10 | 3 | 10 | 87 | 62.1 | 51.7 |
| CS-6/HA-10 | 6 | 10 | 84 | 55.3 | 47.0 |
| CS-9/HA-10 | 9 | 10 | 81 | 50.3 | 45.8 |
| CS-12/HA-10 | 12 | 10 | 78 | 47.0 | 33.8 |
Preparation of CS–HA/PMMA composites. The CS–HA/PMMA bone composite was synthesized by free radical polymerization. An MMA solution with the photoinitiator, BPO, and coinitiator, BHET, was prepared as follows: photoinitiator BPO (1% w/w) and co-initiator BHET (1% w/w) were dissolved in MMA, and stirred for 5 min to achieve a homogenous solution. Then, the resultant solution was injected into the porous CS–HA scaffold rapidly and ultrasonicated for 5 min so that the MMA solution could be dispersed uniformly in the CS–HA scaffold. Free radical polymerization of MMA took place in the porous scaffold at room temperature. The cylindrical CS–HA/PMMA bone substitute composite was obtained after 12 h.
2.3 Characterization
where V1 is the volume of original n-hexane, V2 is the volume of n-hexane after the disc was immersed, which was measured immediately, and V3 was the volume of n-hexane measured after the disc was taken out.
3. Results and discussion
3.1 Morphology and porosity
Investigation of the morphology of the CS–HA scaffolds by SEM (Fig. 1) showed that both the prefrozen method and ratio of chitosan had significant influence on the porosity and microstructure of the scaffolds. The scaffolds that were fabricated using SCM had uniform and well interconnected pores; however, the scaffolds that were fabricated using RCM had disordered and disconnected pores. The sample codes and porosity of each sample are shown in Table 1.The SEM results corresponded to the porosity results well. In Table 1, the porosity of the CS–HA scaffolds that were prefrozen in liquid nitrogen was 33.8%, 45.8%, 47.0%, and 51.7%, which correspond to CS-12/HA-10, CS-9/HA-10, CS-6/HA-10 and CS-3/HA-10, respectively. The porosity of the CS–HA scaffolds that were prefrozen at −40 °C was 47.0%, 50.3%, 55.3%, and 62.1%, which correspond to CS-12/HA-10, CS-9/HA-10, CS-6/HA-10 and CS-3/HA-10, respectively. The porosity of the CS–HA scaffolds decreased with the increase of chitosan content. Both the porosity of the CS–HA scaffolds prepared by SCM and RCM decreased with the increase of chitosan content. The initial volume of the frozen chitosan solutions with different chitosan concentrations were the same. As the chitosan concentration increased, the corresponding volume of chitosan increased, hence the volume of ice decreased. Fig. 1a–d correspond to CS-3/HA-10, CS-6/HA-10, CS-9/HA-10 and CS-12/HA-10 prepared by SCM, respectively. Image e, f, g and h correspond to CS-3/HA-10, CS-6/HA-10, CS-9/HA-10 and CS-12/HA-10 prepared by RCM, respectively. The variation trend of porosity was well in accord with the variation trend of morphology. The CS–HA scaffolds that were prepared using the SCM consisted of a three-dimensional interconnected and ordered pore structure, based on the SEM images (Fig. 1a–d). However, CS–HA scaffolds were prepared using RCM had a random porous architecture (Fig. 1e–h). These results may be due to the dissolution process of chitosan and the ice crystallization process.
The proposed mechanism for the formation of chitosan network structures and the microstructure of the scaffolds are illustrated in Scheme 1 and Fig. 2. Natural chitosan has a complex inter- and intra-molecular hydrogen-bond network, which makes it insoluble in water and leads to the chitosan molecule being curled.31 When chitosan was dissolved in acetic acid, the amino groups on chitosan was protonated. Chitosan was well dispersed in the acetic solution due to strong electrostatic repulsions.32 Ice crystallization consists of crystal nucleation and crystal growth. The ice nucleation rate is determined by the degree of supercooling, whereas the ice growth rate is largely controlled by the rate of heat transfer from the crystal surface to the bulk water.33 When the chitosan solution was placed in a refrigerator, ice started to nucleate and grow. Initially, chitosan chains were pushed together by the advancing ice fronts, which led to interactions such as van der Waals attraction, electrostatic repulsion, and hydrogen bonding. Dissolved chitosan chains tend to be excluded from the ice phase during freezing, since the solubility of a solute in ice is almost negligible.34 At this stage, we propose that individual chitosan assembled into the interconnected pores. As ice continued to grow slowly, these chitosan started to form a three-dimensional aperiodic network structure that then became columnar or lamellar structures later, as shown in Fig. 1a–d. Next, the ice crystals were directly sublimated into water vapour, and solute molecules were left, which became the CS–HA scaffolds.
For RCM, columnar ice phases are preferred to disordered lamellar ice crystals, as shown in Fig. 1. From Fig. 1e–h, the lamellar structure turned into a totally disordered porous structure. Rapid freezing can cause dendrite formation. However, if the velocity of ice growth is fast enough, dendritic structures can break down, which allows solutes to get entrapped in the solid rather than excluded to the inter-dendritic region.35 This is the reason why the scaffold prepared using RCM had disordered and disconnected pores. The porosity of the scaffolds prepared using the SCM were higher than those prepared using the RCM due to the connectivity of the pores in the scaffolds. The scaffold prepared using SCM has a three-dimensional aperiodic network structure, which is internally connected. The scaffold prepared using RCM has a disordered network, which is not internally connected. Therefore, the porosity of the scaffolds prepared using SCM were higher than that prepared using RCM.
3.2 Thermogravimetric analysis
The thermal behaviour of the prepared scaffolds was investigated using thermogravimetric analysis (TGA). The TGA and DTG curves of the CS–HA/PMMA scaffolds and bulk PMMA are shown in Fig. 3. The residual weight ratio of bulk PMMA was 0.3%, which belongs to the residual weight of carbon. The residual weight ratio of the CS–HA/PMMA scaffolds that were prepared using SCM was 11.60% after thermal degradation. The residual weight ratio of CS–HA/PMMA scaffolds that were prepared using the RCM was 13.13% after thermal degradation. The residual weight corresponds to the weight of HA and residual carbon. The thermogravimetric behaviour of the CS–HA/PMMA scaffolds fabricated by different methods was almost the same. As shown in Fig. 3a, bulk PMMA had an onset degradation temperature at 326 °C and lost nearly all weight at 428 °C. The degradation of PMMA consisted of three stages. The first stage (150–200 °C) corresponded to the degradation initiation due to the breaking of weak head to head linkages due to its instability. The second (250–300 °C) stage was due to the degradation initiated by the unsaturated ends of radical polymerized PMMA. The last stage (350 °C) was caused by the random chain scission of PMMA.The degradation behaviour of the CS–HA/PMMA composite was different from that of bulk PMMA. The scaffolds had two degradation stages at 300 °C and 395 °C (Fig. 3b), respectively. The former temperature is ascribed to the part degradation of chitosan and the latter temperature corresponds to the degradation of PMMA. Compared with bulk PMMA, the presence of CS and HA offers a stabilizing effect for PMMA since the onset of degradation occurred at a high temperature. The residual mass at 400 °C for bulk PMMA was nearly 0%, whereas that for the bone composite was still 35.68%. This data implied that the presence of CS and HA hindered the unzipping of PMMA. The retardation effects might be attributed to the interaction between CS and the macro-radicals generated during the degradation process.
The residual mass ratio at 600 °C for CS–HA/PMMA was nearly 10%, which is almost equal to the content of HA before freeze drying. The curves also showed that the prefrozen methods had no significant influence on the thermal behaviour of the prepared composites.
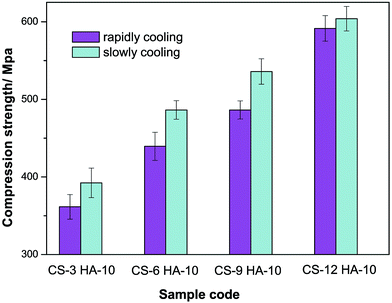
3.3 Mechanical compression testing
The mechanical property of materials plays a vital role in determining the long-term stability of biomaterials. The compression modulus of the different CS–HA/PMMA scaffolds is shown in Fig. 4. This indicates that the compression modulus was significantly influenced by the CS content and prefrozen method. The compression modulus of the scaffolds improved with the increase of chitosan. This was attributed to the uniform dispersion of chitosan in PMMA and the reinforcement of effect of chitosan. Chitosan, which is a rigid and semicrystallized oligomer, played an important role in improving the mechanical strength of the two-continuous phase composites. CS–HA/PMMA scaffolds prepared using SCM had a much higher compression modulus than that of the scaffolds prepared using RCM. During the MMA injecting procedure, the scaffolds prepared using SCM had interconnected pores, hence MMA could combine well with the CS–HA scaffolds. This insured that PMMA had a continuous phase to provide a higher compression modulus. However, the scaffolds prepared using RCM had disconnected pores and the polymerized PMMA did not behave as a continuous phase, which was bad for the material's compression modulus.3.4 XRD
Fig. 5 shows the wide-angle X-ray diffractograms of the CS–HA/PMMA scaffolds. Bone scaffolds prepared using SCM and RCM both showed typical HA peaks. The peak at 2θ = 13° in the XRD could be ascribed to chitosan. Peaks in the XRD spectra could be ascribed to HA with the major peaks at 2θ = 25.95°, 31.83°, 33.02°, 34.11°, and 39.93° corresponding to the (002), (211), (300), (202) and (310) diffraction planes of crystalline HA (JCPDS 9-432), respectively, whereas PMMA appeared more amorphous in morphology than HA. The diffraction pattern of bulk PMMA was consistent with a previous report,36 which showed broad peaks at 14.2° and 29.9°, thus indicating the amorphous state of PMMA (Fig. 5).3.5 In vitro cell cytotoxicity
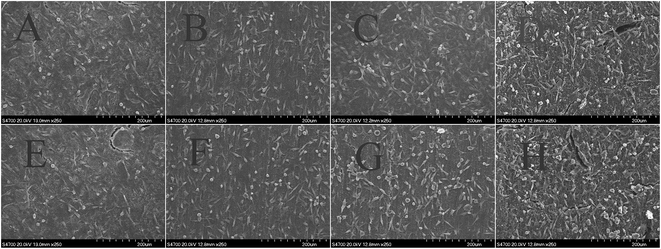
An ideal bone substitute should not release toxic products or produce adverse reactions, which can be evaluated via in vitro cytotoxic tests. The level of toxicity of the CS–HA/PMMA composites cultured for different periods of time towards the viability of fibroblasts cells was evaluated using the ISO10993-5 standard test method of the indirect MTT cytotoxicity assay and the results are presented in Fig. 6. As can be seen, the cell viability data show that the addition of the as-prepared CS–HA/PMMA scaffolds into the cell culture demonstrated no significant toxicity (p > 0.05) to the cell viability when compared with the negative control and the average absorbance values are almost equal to that of the control condition. However, slight reductions in the optical density (OD) values were observed for RCM. This may result due to the disorder of chitosan. It can be seen from Fig. 6 that the average viability of fibroblasts cells cultured on CS–HA/PMMA composites reached above 80%, which meant that fibroblasts cells might occupy all available spaces on the CS–HA/PMMA composites. The obtained results suggest that the CS–HA/PMMA composites were nontoxic to L929 cells.Fig. 7 corresponds to the cell attachment and proliferation studies of L929 cells. (A)–(H) correspond to the SEM images of cell attachment on the prepared bone scaffolds with different chitosan contents and different prefrozen methods. The SEM images show that the biocompatibility and cell proliferation of the bone scaffolds were all good. The cells appeared to adhere on the surface well and exhibited a normal morphology on the surface of the CS–HA/PMMA composites. The images also show that the number of cells increased with the increase of chitosan content in the composites. Chitosan and hydroxyapatite play a role in enhancing the activity of L929 cells. These data indicate that the composite scaffolds showed enhanced biocompatibility with the increase of chitosan content. The biocompatibility and cell proliferation of the bone scaffolds show the same trend. The number of cells on the scaffolds prepared using different prefrozen methods with the same chitosan concentration was almost the same. This also demonstrates that the prefrozen method has no significant influence on the biocompatibility of the CS–HA/PMMA composites.
4. Conclusions
In this study, a series of CS–HA/PMMA composites with good mechanical properties were fabricated using the freeze drying method and free radical polymerization. The prefrozen method had significant influence on the mechanical properties of the materials. The prepared bone scaffolds were characterized using TGA and SEM. The porosity of the CS–HA scaffolds were evaluated and it was shown that the prefrozen method and chitosan concentration had significant influence on the morphology and mechanical property of bone scaffolds. The porosity and compression modulus of CS–HA scaffolds prefrozen at −40 °C were higher than those prefrozen in liquid nitrogen. The porosity of the scaffolds decreased as the chitosan content increased. In contrast, as the chitosan content increased, the compression modulus increased significantly, and cell proliferation also became better. The prefrozen method had no significant influence on the thermal behaviour.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science 382 Foundation of China (Grant No. 21304005) and the Jiangsu Provincial Natural Science 383 Foundation (Grant No. BK20131145).Notes and references
- R. Murugan and S. Ramakrishna, Biomaterials, 2004, 25, 3829 CrossRef CAS PubMed.
- W. R. Moore, S. E. Graves and G. I. Bain, Aust. N. Z. J. Surg., 2001, 71, 354 CrossRef CAS.
- Y. Cheng, X. Luo, J. Betz, G. F. Payne, W. E. Bentley and G. W. Rubloff, Soft Matter, 2011, 7, 5677 RSC.
- J. J. Chris Arts, N. Verdonschot, B. W. Schreurs and P. Buma, Biomaterials, 2006, 27, 1110 CrossRef CAS PubMed.
- J. M. Holzwarth and P. X. Ma, Biomaterials, 2011, 32, 9622 CrossRef CAS PubMed.
- M. C. Gutiérrez, M. Jobbágy, M. L. Ferrer and F. d. Monte, Chem. Mater., 2008, 20, 11 CrossRef.
- M. Fosca, V. S. Komlev, A. Y. Fedotov, R. Caminiti and J. V. Rau, ACS Appl. Mater. Interfaces, 2012, 4, 6202 CAS.
- L. Bi, W. Cheng, H. Fan and G. Pei, Biomaterials, 2010, 31, 3201 CrossRef CAS PubMed.
- S. W. Choi, J. Xie and Y. Xia, Adv. Mater., 2009, 21, 2997 CrossRef CAS PubMed.
- J. Mitra, G. Tripathi, A. Sharma and B. Basu, RSC Adv., 2013, 3, 11073 RSC.
- S. W. Choi, J. Xie and Y. Xia, Adv. Mater., 2009, 21, 2997 CrossRef CAS PubMed.
- L. C. Palmer, C. J. Newcomb, S. R. Kalt, E. D. Spoerke and S. I. Stupp, Chem. Rev., 2008, 108, 4754 CrossRef CAS PubMed.
- L. L. Hench, J. Am. Ceram. Soc., 1998, 18, 1705 Search PubMed.
- L. Chen, J. Hu, J. Ran, X. Shen and H. Tong, RSC Adv., 2015, 5, 56410 RSC.
- F. Scalera, F. Gervaso, K. P. Sanosh, A. Sannino and A. Licciulli, Ceram. Int., 2013, 39, 4839 CrossRef CAS PubMed.
- Q. Wu, X. Zhang, B. Wu and W. Huang, Ceram. Int., 2013, 39, 2389 CrossRef CAS PubMed.
- L. Jiang, Y. Li, X. Wang, L. Zhang, J. Wen and M. Gong, Carbohydr. Polym., 2008, 74, 680 CrossRef CAS PubMed.
- J. R. Woodard, A. J. Hilldore, S. K. Lan, C. J. Park, A. W. Morgan, J. A. Eurell, S. G. Clark, M. B. Wheeler, R. D. Jamison and A. J. Wagoner Johnson, Biomaterials, 2007, 28, 45 CrossRef CAS PubMed.
- M. a. C. Gutiérrez, Z. Y. Garcia-Carvajal, M. a. J. Hortiguela, L. Yuste, F. Rojo, M. a. L. Ferrer and F. del Monte, J. Mater. Chem., 2007, 17, 2992 RSC.
- L. Qian and F. H. Zhang, J. Chem. Technol. Biotechnol., 2011, 86, 172 CrossRef CAS PubMed.
- G. Jo, W.-K. Hong, J. I. Sohn, M. Jo, J. Shin, M. E. Welland, H. Hwang, K. E. Geckeler and T. Lee, Adv. Mater., 2009, 21, 2156 CrossRef CAS PubMed.
- S. Deville, Science, 2006, 311, 515 CrossRef CAS PubMed.
- C. Jiang, Z. Wang, X. Zhang, X. Zhu, J. Nie and G. Ma, RSC Adv., 2014, 4, 41551 RSC.
- P. Jithendra, A. M. Rajam, T. Kalaivani, A. B. Mandal and C. Rose, ACS Appl. Mater. Interfaces, 2013, 5, 7291 CAS.
- J. Han, G. Ma and J. Nie, Mater. Sci. Eng., C, 2011, 31, 1278 CrossRef CAS PubMed.
- Y. Zhang, J. R. Venugopal, A. El-Turki, S. Ramakrishna, B. Su and C. T. Lim, Biomaterials, 2008, 29, 4314 CrossRef CAS PubMed.
- T. Almeida, B. J. M. Leite Ferreira, J. Loureiro, R. N. Correia and C. Santos, Mater. Sci. Eng., C, 2011, 31, 658 CrossRef CAS PubMed.
- R. Kang, Y. Luo, L. Zou, L. Xie, H. Lysdahl, X. Jiang, C. Chen, L. Bolund, M. Chen, F. Besenbacher and C. Bünger, RSC Adv., 2014, 4, 5734 RSC.
- M. S. Fernandez, J. I. Arias, M. J. Martinez, L. Saenz, A. Neira-Carrillo, M. Yazdani-Pedram and J. L. Arias, J. Tissue Eng. Regener. Med., 2012, 6, 497–504 CrossRef CAS PubMed.
- C. Jiang, Z. Wang, X. Zhang, X. Zhu, J. Nie and G. Ma, RSC Adv., 2014, 4, 41551 RSC.
- J. R. Woodard, A. J. Hilldore, S. K. Lan, C. J. Park, A. W. Morgan, J. A. Eurell, S. G. Clark, M. B. Wheeler, R. D. Jamison and A. J. Wagoner Johnson, Biomaterials, 2007, 28, 45 CrossRef CAS PubMed.
- J. Wu and J. C. Meredith, ACS Macro Lett., 2014, 3, 185 CrossRef CAS.
- M. Matsumoto, S. Saito and I. Ohmine, Nature, 2002, 416, 409 CrossRef CAS PubMed.
- K. M. Pawelec, A. Husmann, S. M. Best and R. E. Cameron, Appl. Phys. Rev., 2014, 1, 021301 Search PubMed.
- M. Akyurt, G. Zaki and B. Habeebullah, Energy Convers. Manage., 2002, 43, 1773 CrossRef CAS.
- Y. Li, B. Zhang and X. Pan, Compos. Sci. Technol., 2008, 68, 1954 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |