From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets†
Abstract
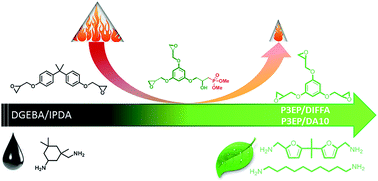
In this work, phloroglucinol was used as a renewable resource to prepare an epoxy monomer and phosphorus containing reactive flame retardant (FR). These building blocks were reacted with diamines to obtain partly or fully bio-based flame retardant epoxy resins. It was highlighted that the glass transition temperature of the materials was tightly related to the functionality of the reactive monomers and the resulting crosslink density. Thermal stability and char yield of the thermosets seems to be mainly governed by the aromaticity of the monomers, the linking rate of the aromatic ring and the phosphorus content. Phosphorus FR are more efficient in intrinsically poorly charring matrices. It was evidenced that the flammability of bio-based epoxies can be monitored by two strategies: (i) choosing bio-based monomers with high charring ability and low combustion energy, (ii) incorporating bio-based phosphorus-containing reactive FR in the polymer network.

 Please wait while we load your content...
Please wait while we load your content...