Facile synthesis of (Ni,Co)@(Ni,Co)xFe3−xO4 core@shell chain structures and (Ni,Co)@(Ni,Co)xFe3−xO4/graphene composites with enhanced microwave absorption†
Abstract
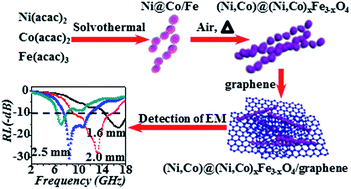
A facile strategy was developed to synthesize a series of multiple metal@metal oxide hybrid structures, such as (Ni,Co)@(Ni,Co)xFe3−xO4, Ni@NixFe3−xO4, Co@CoxFe3−xO4, and (Ni,Co)@NixCo3−xO4. The (Ni,Co)@(Ni,Co)xFe3−xO4 core@shell chain structures display enhanced microwave absorption performance compared with other hybrid structures. The (Ni,Co)@(Ni,Co)xFe3−xO4 demonstrates not only a combination of the distinct component properties but also a synergy between different components. By introducing graphene into a composite system—(Ni,Co)@(Ni,Co)xFe3−xO4/graphene, much better microwave absorption performance can be achieved. Its reflection loss value can reach −30.34 dB at 8.48 GHz with a thickness of 2.5 mm and its absorption bandwidth with the reflection loss below −10 dB is about 7 GHz from 9.12 to 16.08 GHz with a thickness of 2.0 mm. Our study illustrates the feasibility that microwave absorption materials with strong absorption properties as well as being lightweight and having a broad absorption frequency range can be rationally designed by constructing hybrid structures and composites.

 Please wait while we load your content...
Please wait while we load your content...