Modification of Eu3+–beta-diketonate complex-intercalated LAPONITE® with a terpyridine-functionalized ionic liquid
Yalan Yao,
Zhiqiang Li and
Huanrong Li*
School of Chemical Engineering and Technology Hebei University of Technology, GuangRong Dao 8, Hongqiao District, Tianjin 300130, P. R. China. E-mail: lihuanrong@hebut.edu.cn
First published on 23rd July 2015
Abstract
A lanthanide complex-based organic–inorganic hybrid material with intense luminescence has been obtained by modifying Eu3+–beta-diketonate complex-intercalated LAPONITE® with a terpyridine moiety-functionalized ionic liquid. Remarkable luminescence enhancement as well as improved thermal- and photo-stability were observed after modification with the functional ionic liquid. The ionic liquid is believed to coordinate to and sensitize Eu3+ ions as well as decrease proton concentration on LAPONITE® surfaces.
1. Introduction
To date, lanthanide complex based luminescent inorganic–organic hybrid materials have attracted the attention of researchers across various disciplines due to their rich applications, such as display, lighting, optical devices and medical technology.1–10 Such luminescent materials typically are prepared by doping lanthanide complexes into various matrices including zeolites,11–13 polymers,14 mesoporous silica,15 titania,16,17 xerogels,18 and clay materials.19,20 As the existing literature illustrates, lanthanide complexes encapsulated in a matrix lead to better optical properties and higher stability compared with the individual lanthanide complexes. As synthesized clay, LAPONITE® has a layered structure with expandable interlayer spaces and favourable cation-exchange capacity, which can be completely exfoliated in water.21–23 These inherent properties make it a suitable host for incorporation of lanthanide complexes in aqueous solution to form luminescent hybrid materials. However, abundant acidic sites exist on the surface of the individual delaminated platelets of LAPONITE® in water, which have negative influence on the luminescence efficiency.24,25 For instance, the luminescence efficiency of terbium complexes (Tb(bpy)23+) on the platelets of LAPONITE® appears to be rather low.25 Fortunately, Yang et al. observed a significant increase in the luminescence efficiency of hybrids consisting of Eu3+–beta-dikeonate and LAPONITE® after adding imidazolium salt, which has been ascribed to the decreasing of the proton activity on clay surface by the added imidazolium salt that act through a mechanism of synergic effect of ion exchange and neutralization.26 Other ionic liquids even displaying acidity in water can also enhance the luminescence of such kind of hybrid materials due to their capacity of removing proton via ion exchanges.26Encouraged by this, herein, we report the luminescence enhancement of hybrid materials consisting of Eu3+ (ttan) complex (tta = 2-thenoyltrifluoroacetone) and the inorganic matrix LAPONITE® upon modification with a terpyridine moiety-containing ionic liquid (tpy-IL),27 which is believed to act both as a sensitizer for Eu3+ ions and as an proton exchanger for decreasing the proton concentration on LAPONITE® surfaces. In addition, improved thermal- and photo-stability of the tpy-IL modified hybrid materials can also be observed.
2. Results and discussion
LAPONITE® (chemical composition Na0.7[Si8Mg5.5Li0O20(OH)4]) is a layered smectite-type clay with stacked platelets average dimension of 1 nm × 25 nm. It is well known that it is completely delaminated to individual disks to form transparent solution after swelling in water. Each single platelet contains roughly 1500 unitary cells (u.c.). In consequence, counter cations such as Na+ are accessible to ion exchange and organic species can be easily inserted in the interlayer.22,28 According to the supplier, LAPONITE® has a cation exchange capacity (CEC) of 50–60 meq/100 g, i.e. about 55–65% of the existing Na+ ions.20,21,29,30 The mean layer charge of LAPONITE® determined using the n-alkylammonium method is 0.346.31–33 In this study, the intercalation of Eu3+–beta-diketonate complexes within the LAPONITE® was achieved by a two-step procedure according to our previous work. Firstly, material of Eu3+@LA were prepared by substituting the positive Na+ ions with Eu3+ via ion exchanges in aqueous solution, followed by addition of ethanol solution of tta. We find 80% of the available Na+ ions are exchanged by Eu3+ ions (through analyzing the supernatant titration against EDTA). As a result, the final experimentally determined tta and Eu3+ loading per u.c. under this condition is ∼1 and ∼0.352, respectively.26 The presence of the tta ligand can be detected by UV-vis absorption according to the procedure.27 As shown in Fig. 1c, the Eu3+(ttan)@LA exhibited characteristic emission of Eu3+ when irradiated by near UV light, this phenomenon indicate the formation of Eu3+–beta-diketonate complexes in between the interlayers of LAPONITE®. After the insertion of tpy-IL, the luminescence enhancement was observed by naked eyes in the obtained Eu3+(ttan)@LA/tpy-IL. The influence of the ionic liquid in the hybrid materials is especially obvious, which shows bright red emission (Fig. 1d).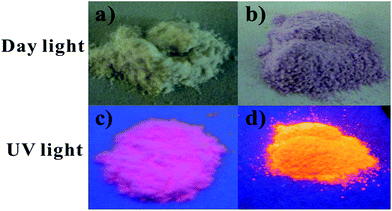 | ||
| Fig. 1 Digital photos of the samples; (a) Eu3+(ttan)@LA (day light), (b)Eu3+(ttan)@LA/tpy-IL (day light); (c) Eu3+(ttan)@LA (UV light), (d)Eu3+(ttan)@LA/tpy-IL (UV light). | ||
2.1 X-ray diffraction
The formation of luminescent hybrid materials based on LAPONITE® was confirmed by the powder X-ray diffraction (XRD) (Fig. 2). All the samples exhibit a somewhat broad diffraction pattern, indicating a rather pronounced stacking disorder and the small crystallites particle size of the samples.34 The broad diffraction pattern at approximately 2θ = 5.89° is attributed to the (001) crystal plane or the basal spacing of LAPONITE®.19 | ||
| Fig. 2 XRD patterns of (a) LA, (b) Eu3+(ttan)@LA and (c) Eu3+(ttan)@LA/tpy-IL (left), idealized structure formula of LAPONITE® (right). | ||
As shown in Fig. 2b, the basal spacing of LAPONITE® expanded from approximately 15 Å to 16.4 Å after the accommodation of Eu3+–beta-diketonate complexes, and implied that at least a proportion of tta complexes were intercalated within the interlayers. The basal spacing of LAPONITE® in Eu3+(ttan)@LA/tpy-IL increased from approximately 15 Å to 18.5 Å (Fig. 2c), indicating that tpy-IL has been located the interlayer space of LAPONITE®. Nevertheless, the location of guest complexes on external adsorption sites on LAPONITE® could not be excluded.
2.2 FT-IR spectra and SEM
The modification of tpy-IL with Eu3+(ttan)@LA can be further confirmed by the FTIR spectra. Fig. 3 displays the FTIR spectra of Eu3+(ttan)@LA (a) and Eu3+(ttan)@LA/tpy-IL (b). Bands centered at about 1607, 1587, and 1563 cm−1 are assigned to the imidazole ring and the pyridine ring of tpy-IL.35,36 Upon modification of tpy-IL with the hybrid material Eu3+(ttan)@LA, these bands appears in spectrum (b) and the shift of some absorption bands corresponding to the terpyridine moieties also can be observed, which indicates a successful formation of Eu3+(ttan)@LA/tpy-IL. The morphology of the Eu3+(ttan)@LA and Eu3+(ttan)@LA/tpy-IL was characterized by SEM experiment. As shown in Fig. 4a, a number of uniform size nanoparticles with an average diameter of 40–50 nm were observed in SEM image. Similar morphology with larger size nanoparticles was observed in the SEM image of Eu3+(ttan)@LA/tpy-IL (Fig. 4b).2.3 Optical properties
We find that the luminescence performance of Eu3+(ttan)@LA/tpy-IL is significant influenced by the initial addition amount of tpy-IL. The luminescence intensity of Eu3+(ttan)@LA/tpy-IL increased gradually with increasing amount of tpy-IL, which reached its maximum when the quality ratio of tpy-IL to Eu3+(ttan)@LA was set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 5). Therefore, the initial amount of tpy-IL to Eu3+(ttan)@LA was maintained at 1
1 (Fig. 5). Therefore, the initial amount of tpy-IL to Eu3+(ttan)@LA was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the following experiments. The amount of tpy-IL actually loaded on the Eu3+(ttan)@LA/tpy-IL hybrid materials was determined by elemental analysis. We found that the loaded amount of tpy-IL is about 0.225 per u.c.
1 in the following experiments. The amount of tpy-IL actually loaded on the Eu3+(ttan)@LA/tpy-IL hybrid materials was determined by elemental analysis. We found that the loaded amount of tpy-IL is about 0.225 per u.c.
 | ||
| Fig. 5 Luminescence intensity at 612 nm versus the amount of tpy-IL initially added to Eu3+(ttan)@LA. | ||
Further support is gained from the optical spectra as shown in Fig. 6. Both Eu3+(ttan)@LA and Eu3+(ttan)@LA/tpy-IL materials show similar excitation and emission spectra, the excitation spectra are composed of a broad band from 200 to 400 nm resulting from the absorption of ligands. The excitation of the Eu3+(ttan)@LA is rather low over the monitored spectral range. Nevertheless, introduction of tpy-IL into the Eu3+(ttan)@LA materials leads to a remarkable excitation enhancement peaked at approximately 370 nm. Characteristic luminescence of Eu3+ located at 579 (5D0 → 7F0), 592 (5D0 → 7F1), 612 (5D0 → 7F2), 652 (5D0 → 7F3) and 698 (5D0 → 7F4) nm was observed both in Eu3+(ttan)@LA and Eu3+(ttan)@LA/tpy-IL under excited at 350 nm (Fig. 6b). However, Eu3+(ttan)@LA show relatively weak characteristic luminescence of Eu3+, obvious improvement in luminescence intensity is observed upon the incorporation of tpy-IL. Apparently, the spectrum is dominated by the hypersensitive transition 5D0 → 7F2, resulting in bright red luminescence. The integrated intensity of 5D0 → 7F2 transition of Eu3+(ttan)@LA/tpy-IL was 10 times than that of Eu3+(ttan)@LA.
The remarkable luminescent enhancement in Eu3+(ttan)@LA/tpy-IL can be explained as follows. The addition of tpy-IL can fully protect Eu3+ ions from the water molecules quenching and remove the abundant protons on the platelets. The number of water molecules in the coordination sphere of Eu3+ was evaluated based on the emission spectra and the life time of the 5D0 state of the Eu3+ ions according to the method described elsewhere.37 The coordinated water molecules in Eu3+(ttan)@LA was ∼3, while the number of water molecules coordinated to the europium ions in the hybrid material Eu3+(ttan)@LA/tpy-IL was calculated to be 0.8. This means that most of the water molecules have been shielded from the first coordination sphere of the Eu3+ ions by the synergistic coordination of organic ligand combined with the functionalized ionic liquid.
In addition, the intensity ratio of I(5D0 → 7F2)/I(5D0 → 7F1) is increased from 6 to 12, indicating the asymmetric coordination field around Eu3+ ions, which is due to the strong coordination interaction appearing between the ligands and the Eu3+ ions.38 Moreover, obvious stark splitting (3 lines 5D0 → 7F2) can be seen, which further indicates the low-symmetry site occupied by Eu3+ ion and a strong ligand field around Eu3+ ion. On the other hand, it has been well-documented that the acid environment has significant influence on the luminescence performances of Eu3+–beta-diketonate complexes.25 TTA ligand can be protonated under acidic environment, which competes with full coordination to Eu3+ ions.26 We propose that the protons on the platelets can be removed by addition of tpy-IL through a mechanism of synergic effect of ion exchange and neutralization. This can be supported by the following observations, the suspension of Eu3+(ttan)@LA shows a decrease in pH from ∼7.3 to ∼6.6 after addition of tpy-IL, indicating the releasing of protons from the surface of LAPONITE® by exchanging H+ with positively charged of tpy-IL. In order to further exploring the luminescence properties of both hybrid materials, the typical decay curves were measured (Fig. 6c). The observed lifetime of Eu3+(ttan)@LA/tpy-IL (0.61 ms) is longer than Eu3+(ttan)@LA (0.23 ms), which is in coincidence with the results of emission spectra. As expected, the modified materials yield about 7-fold increase in luminescence quantum efficiency.
2.4 Thermal- and photo-stability
Drawbacks such as low thermal39,40 and photochemical stability41 and poor mechanical limit the full exploitation of lanthanide complex in practical application. Hybridize the complexes with stable matrices provide an effective way to overcome the drawbacks.12,18,42–45 In the present work, the thermal-and photo-stability of both materials were studied.We performed thermogravimetric analysis (TGA) to compare the thermal stability of the Eu3+(ttan)@LA and the Eu3+(ttan)@LA/tpy-IL. Three decomposition stages can be observed in the TG curve of Eu3+(ttan)@LA (Fig. 7a). The first weight loss (6%) below 150 °C could be attributed to the removal of water. The second step, there is a 5% mass loss between 150–330 °C, which corresponds to the dissociation of the tta component. Beyond 330 °C, the LAPONITE® structure also decomposes with a gradual weight loss of 7% and a plateau is developed above 1000 °C. For comparison, the thermal analysis profile of Eu3+(ttan)@LA/tpy-IL is shown in Fig. 7b. Below 150 °C, no obvious weight loss is observed for the TG curve, implying less water molecules coordinated in the complex. This is probably due to the formation of Eu3+(ttan) with tpy-IL. The weight loss (28%) between 200 and 480 °C is possibly associated with the degradation of organic moieties from tta and tpy-IL. The LAPONITE® decomposes process is concentrated at narrow temperature range of 480–800 °C, this phenomenon reflected a more regular and orderly structure in Eu3+(ttan)@LA/tpy-IL, which is believed to be ascribed to the enhanced luminescence properties after addition of tpy-IL. As concluded, Eu3+(ttan)@LA/tpy-IL exhibited a higher thermal stability than Eu3+(ttan)@LA.
In addition, the thermo-stability of the hybrid materials with and without tpy-IL also was examined by heating at 150 °C under air. The time-dependence profiles of the 5D0/7F2 transition integrated intensity are shown in Fig. 8. We observed drastic decrease of emission intensity of Eu3+(ttan)@LA without tpy-IL protection (36%) after 20 h. In contrast, few decrease of the emission intensity of Eu3+(ttan)@LA/tpy-IL was observed, 90% of its original integrated intensity was remained. Photo-stability was investigated by exposing the hybrid materials under ultraviolet lamp. The emission spectra curve of Eu3+(ttan)@LA and Eu3+(ttan)@LA/tpy-IL exposed to UV irradiation for 10 h are shown in Fig. 9. A ∼45% decrease in the 5D0/7F2 transition integrated intensity is observed for Eu3+(ttan)@LA while the value is only ∼8% for Eu3+(ttan)@LA/tpy-IL. In summary, pronounced improved thermal- and photo-stability is achieved by incorporation tpy-IL to the hybrid materials. As we can see from the above results, not only because unique properties of the ionic liquid (tpy-IL), but also coordinating ability between europium(III) ions and the ligand plays a crucial role in improving the stability of the hybrid materials.
 | ||
| Fig. 8 Changes of the integrated intensity of 5D0/7F2 transition after 150 °C heat treatment (holding 0 h, 5 h, 10 h, 15 h, 20 h); —Eu3+(ttan)@LA; —Eu3+(ttan)@LA/tpy-IL. | ||
 | ||
| Fig. 9 Emission spectra of (a) Eu3+(ttan)@LA and (b) Eu3+(ttan)@LA/tpy-IL after exposed to UV irradiation (λex = 365 nm) (holding 0 h, 10 h). | ||
3. Experimental
3.1 Materials
LAPONITE® and the alkylamines were purchased from Rockwood Additives Ltd and used as received without further purification. 2-Thenoyltrifluoroacetone (tta) was purchased from Aldrich. Europium chloride (EuCl3·6H2O) was prepared by dissolving Eu2O3 into concentrated hydrochloride acid (37%). The excess acid was removed by addition of ethanol followed by successive fuming. The organic salt containing terpyridine moieties (tpy-IL) was synthesized according to the reported procedure.273.2 Characterizations
X-ray powder diffraction studies were completed with an X-ray powder diffractometer (BRUKER D8 Focus) employing Cu Kα radiation (λ = 1.5418 Å), operating at 40 kV and 40 mA. Infrared (IR) spectra were obtained with a Bruker Vector 22 spectrometer by using KBr pellets for solid samples ranging from 400 to 4000 cm−1. The UV-Vis spectra were recorded on a VARIAN CARY 50 UV-Vis spectrophotometer. Elemental analysis was performed on a Flash EA 1112. SEM images were obtained from a Nova Nano SEM450 at an acceleration voltage of 15 kV. Luminescence spectra were measured on an Edinburgh Instruments model FLS920P spectrometer, with a 450 W Xe lamp as the steady-state excitation source, a double-excitation monochromator (1800 lines mm−1), an emission monochromator (600 lines mm−1), and a semiconductor cooled Hamamatsu model RMP928 photomultiplier tube. Layer charges were estimated from the n-alkylammonium ion-exchange technique32 and the exchange procedure were operated according to this procedure.333.3 Preparation of Eu3+(ttan)@LA
On addition of 5 mL EuCl3·6H2O (0.1 mol L−1) solutions to 5 wt% aqueous dispersions of LAPONITE® (0.5 g), the mixture was stirred at 80 °C for 24 h. The product (denoted as Eu3+@LA) was collected by centrifugation and washed with deionized water several times. The Eu3+@LA was dispersed in 10 mL water; 0.15 g of tta dissolved in 5 mL of EtOH was then added. After vigorous sonication of the mixture for 2 h, the product was collected by centrifugation, washed with EtOH, and dried at 80 °C overnight. The product was denoted as Eu3+(ttan)@LA. CHN analysis of Eu3+(ttan)@LA: C 9.00%, H 3.68%.3.4 Modification of Eu3+(ttan)@LA with tpy-IL
The Eu3+(ttan)@LA was mixed with aqueous solution of tpy-IL in accordance with suitable molar ratio, the mixture was sonicated for 1 h. The product (Eu3+(ttan)@LA/tpy-IL) was then recovered by centrifugation, washed three times with water and dried at 80 °C. Characteristic bands of tpy-IL was observed in the FTIR spectra of Eu3+(ttan)@LA/tpy-IL, which indicate the coordination of tpy-IL with Eu3+ ions. CHN analysis of Eu3+(ttan)@LA/tpy-IL: C 14.15%, N 1.43%, H 3.61%.4. Conclusions
In conclusion, we have observed a remarkable increase of luminescence efficiency of a inorganic–organic hybrid material, obtained by loading of Eu3+–beta-diketonate complexes into the LAPONITE® interlayers by a two-step procedure, after modification with tpy-IL. The present of tpy-IL can fully protect Eu3+ ions from the water molecule quenching together with remove the abundant protons on the platelets are believed to be ascribed to the luminescence enhancement. The unique luminescence properties of the hybrid material, together with its good stability and processability, make it highly promising candidate for fabrication of different kinds of optical devices.Acknowledgements
Financial support by the National Key Basic Research Program (2012CB626804), the National Natural Science Foundation of China (20901022, 21171046, 21271060, and 21236001), the Tianjin Natural Science Foundation (13JCYBJC18400), the Natural Science Foundation of Hebei Province (No. B2013202243), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, IRT1059), and Educational Committee of Hebei Province (2011141, LJRC021) is gratefully acknowledged.Notes and references
- T. Jüstel, H. Nikol and C. Ronda, Angew. Chem., Int. Ed., 1998, 37, 3084–3103 CrossRef.
- C. Feldmann, T. Jüstel, C. R. Ronda and P. J. Schmidt, Adv. Funct. Mater., 2003, 13, 511–516 CrossRef CAS PubMed.
- A. de Bettencourt-Dias, Dalton Trans., 2007, 2229–2241 RSC.
- A. L. Jenkins, O. M. Uy and G. M. Murray, Anal. Chem., 1999, 71, 373–378 CrossRef CAS.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- F. Olivero, F. Carniato, C. Bisio and L. Marchese, J. Mater. Chem., 2012, 22, 25254–25261 RSC.
- H. Li, M. Li, Y. Wang and W. Zhang, Chem.–Eur. J., 2014, 20, 10392–10396 CrossRef CAS PubMed.
- S. Lis, Z. Piskula, K. Staninski, S. Tamaki, M. Inoue and Y. Hasegawa, J. Rare Earths, 2008, 26, 185–191 CrossRef.
- W. Yanli, X. Xianzhu, L. Qianlan, Y. Ruchun, D. Haixin and X. Qiang, J. Rare Earths, 2015, 33, 529–534 CrossRef.
- Z. Piskuła, J. Czajka, K. Staninski and S. Lis, J. Rare Earths, 2014, 32, 221–225 CrossRef.
- L. Chen and B. Yan, Spectrochim. Acta, Part A, 2014, 131, 1–8 CrossRef CAS PubMed.
- Y. Wada, M. Sato and Y. Tsukahara, Angew. Chem., Int. Ed., 2006, 45, 1925–1928 CrossRef CAS PubMed.
- M. Alvaro, V. Fornés, S. García, H. García and J. Scaiano, J. Phys. Chem. B, 1998, 102, 8744–8750 CrossRef CAS.
- M. I. Stich, S. Nagl, O. S. Wolfbeis, U. Henne and M. Schaeferling, Adv. Funct. Mater., 2008, 18, 1399–1406 CrossRef CAS PubMed.
- M. Ogawa, T. Nakamura, J.-i. Mori and K. Kuroda, J. Phys. Chem. B, 2000, 104, 8554–8556 CrossRef CAS.
- T. Nakato, K. Kusunoki, K. Yoshizawa, K. Kuroda and M. Kaneko, J. Phys. Chem., 1995, 99, 17896–17905 CrossRef CAS.
- B. Yan and Q.-M. Wang, Cryst. Growth Des., 2008, 8, 1484–1489 CAS.
- L. D. Carlos, R. A. Ferreira, V. d. Z. Bermudez and S. J. Ribeiro, Adv. Mater., 2009, 21, 509–534 CrossRef CAS PubMed.
- M. Lezhnina, E. Benavente, M. Bentlage, Y. Echevarria, E. Klumpp and U. Kynast, Chem. Mater., 2007, 19, 1098–1102 CrossRef CAS.
- M. E. Hagerman, S. J. Salamone, R. W. Herbst and A. L. Payeur, Chem. Mater., 2003, 15, 443–450 CrossRef CAS.
- M. M. Lezhnina, T. Grewe, H. Stoehr and U. Kynast, Angew. Chem., Int. Ed., 2012, 51, 10652–10655 CrossRef CAS PubMed.
- D. W. Thompson and J. T. Butterworth, J. Colloid Interface Sci., 1992, 151, 236–243 CrossRef CAS.
- T. Felbeck, M. Lezhnina, U. Resch-Genger and U. Kynast, J. Lumin. DOI:10.1016/j.jlumin.2014.11.039.
- S. M. Auerbach, K. A. Carrado and P. K. Dutta, Handb. Layered Mater., CRC Press, 2004 Search PubMed.
- M. Lezhnina, M. Bentlage and U. Kynast, Opt. Mater., 2011, 33, 1471–1475 CrossRef CAS PubMed.
- D. Yang, Y. Wang, Y. Wang, Z. Li and H. Li, ACS Appl. Mater. Interfaces, 2015, 7, 2097–2103 CAS.
- D. Wang, H. Wang and H. Li, ACS Appl. Mater. Interfaces, 2013, 5, 6268–6275 CAS.
- R. A. Caruso, A. Susha and F. Caruso, Chem. Mater., 2001, 13, 400–409 CrossRef CAS.
- J. I. Dawson and R. O. Oreffo, Adv. Mater., 2013, 25, 4069–4086 CrossRef CAS PubMed.
- T. E. Mallouk and J. A. Gavin, Acc. Chem. Res., 1998, 31, 209–217 CrossRef CAS.
- G. Lagaly, Layer Charge Characteristics of, 1994, vol. 2, pp. 1–46 Search PubMed.
- A. Olis, P. Malla and L. Douglas, Clay Miner., 1990, 25, 39–50 CAS.
- A. Czímerová, J. Bujdák and R. Dohrmann, Appl. Clay Sci., 2006, 34, 2–13 CrossRef PubMed.
- B. V. Lotsch and G. A. Ozin, ACS Nano, 2008, 2, 2065–2074 CrossRef CAS PubMed.
- P. Zhang, Y. Wang, H. Liu and Y. Chen, J. Mater. Chem., 2011, 21, 18462–18466 RSC.
- R. López, D. Villagra, G. Ferraudi, S. Moya and J. Guerrero, Inorg. Chim. Acta, 2004, 357, 3525–3531 CrossRef.
- M. Fernandes, V. de Zea Bermudez, R. Sá Ferreira, L. Carlos, A. Charas, J. Morgado, M. M. Silva and M. J. Smith, Chem. Mater., 2007, 19, 3892–3901 CrossRef CAS.
- S. Tang, A. Babai and A. V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7631–7634 CrossRef CAS PubMed.
- Y. Wang, H. Li, L. Gu, Q. Gan, Y. Li and G. Calzaferri, Microporous Mesoporous Mater., 2009, 121, 1–6 CrossRef CAS PubMed.
- D. Yang, J. Wang and H. Li, Dyes Pigm., 2015, 118, 53–57 CrossRef CAS.
- T. Pagnot, P. Audebert and G. Tribillon, Chem. Phys. Lett., 2000, 322, 572–578 CrossRef CAS.
- J. Feng and H. Zhang, Chem. Soc. Rev., 2013, 42, 387–410 RSC.
- K. Lunstroot, K. Driesen, P. Nockemann, L. Viau, P. H. Mutin, A. Vioux and K. Binnemans, Phys. Chem. Chem. Phys., 2010, 12, 1879–1885 RSC.
- L. Carlos and R. S. Ferreira, in Hybrid Nanocomposites for Nanotechnology, Springer, 2009, pp. 509–586 Search PubMed.
- C. Peng, H. Zhang, J. Yu, Q. Meng, L. Fu, H. Li, L. Sun and X. Guo, J. Phys. Chem. B, 2005, 109, 15278–15287 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




