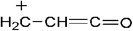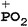Flame retardant and anti-dripping properties of polylactic acid/poly(bis(phenoxy)phosphazene)/expandable graphite composite and its flame retardant mechanism†
Xiaowei Mu,
Bihe Yuan,
Weizhao Hu,
Shuilai Qiu,
Lei Song* and
Yuan Hu*
State Key Laboratory of Fire Science, University of Science and Technology of China, Hefei 230026, China. E-mail: yuanhu@ustc.edu.cn; leisong@ustc.edu.cn; Fax: +86-551-63601664; Tel: +86-551-63601664
First published on 4th September 2015
Abstract
Flame retardant polylactic acid (PLA) composites with poly(bis(phenoxy)-phosphazene) and expandable graphite are prepared by melt blending. Limiting oxygen index, UL-94 vertical burning test, cone calorimeter and thermogravimetric analysis are applied to characterize the flame retardant properties and thermal stability of PLA composites. This flame retardant system shows improved thermal stability, flame retardancy, synergy effect and anti-dripping performance. Raman spectroscopy, X-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy are employed to investigate the chemical structure and composition of the residual char of flame retardant PLA composites after cone calorimeter tests. The residual char is composed of graphite and phosphorus-containing materials. The gaseous phase mechanism of the flame retardant system is investigated with thermogravimetry/Fourier transform infrared spectroscopy and mass spectrometry. The radical species, such as C6H5OP˙, C6H5O˙ and PO2˙, are detected in the gaseous products of PLA composites. Thus, the flame retardant system exhibits both condensed and gas phase flame retardant action in the PLA composites.
1. Introduction
Recently, researchers have paid more and more attention to biodegradable polymers, such as starch polymer, cellulosic polymer and polylactic acid (PLA), due to the shortage of petroleum resources which are the main raw materials of petrochemical plastic. With the advancements in manufacturing technology, PLA has become one of the most available biodegradable polymers owing to its good mechanical properties, high transparency, processability, biocompatibility and biodegradability.1 Now PLA is mainly used as packaging materials, coatings, biomedical materials, etc.2–5 However, just like petrochemical plastic, PLA also shows the drawback of inflammability, which limits its application in electronic and automotive industry.6 Therefore, it is significant to improve flame retardant properties of PLA.PLA is susceptible to ignition with severe dripping and its limiting oxygen index (LOI) value is as low as 21.0%.7 The most common method to endow polymers with flame retardancy is incorporation of flame retardants. However, halogen-containing flame retardants usually produce corrosive and toxic gases during the combustion. Considering the health and environment risks, the halogen-based flame retardants are replaced by halogen-free compounds gradually. Many efforts have been directed towards developing effective non-halogenated flame retardants for PLA. Zhan et al.8 synthesized an intumescent flame retardant (IFR) of spirocyclic pentaerythritol bisphosphorate diphosphoryl melamine (SPDPM). When 25 wt% SPDPM was added into PLA, a LOI value of 38.0% and UL-94 V-0 rating without dripping were obtained in PLA composite. The addition of 15 wt% SPDPM only reached UL-94 V-2 rating with slightly dripping. Tang et al.9 studied the effect of rare earth hypophosphite salts on the fire performance of PLA. UL-94 V-0 rating was obtained by the incorporation of 30 wt% rare earth hypophosphite salts (lanthanum hypophosphite and cerium hypophosphite). However, dripping phenomenon was still observed. Expandable graphite (EG) is another kind of effective IFR for various polymers. Its structure consists of graphite layers within which a chemical compound is intercalated, generally an oxidizing acid (e.g. H2SO4, HNO3). Abundant volume increase of EG is induced by heating and then protective foamed graphite is produced on the polymer surface.10 Wei et al.10 investigated the flame retardant and thermal behavior of PLA/EG composites. UL-94 V-0 rating with dripping was reached at 10 wt% loading.
Due to its polyester chemical structure and degradation behavior, the combustion of PLA is accompanied by serious dripping. Melt dripping will not only broaden the burning surface area which may increase the flame intensity, but also may cause secondary damage during the combustion.11 It is challenge to achieve high flame retardancy and non-dripping for PLA composites simultaneously by low loading of flame retardants. Furthermore, the addition of high amount of flame retardant may deteriorate mechanical properties and processability of polymers and increase the cost of the products. Thus, it is of great importance to develop efficient and environmentally friendly flame retardant for PLA.
In this work, flame retardant PLA composites were prepared by incorporating of EG with a kind of phosphazene polymer, poly(bis(phenoxy)phosphazene) (trade name: SPB-100).12 Polyphosphazene is a kind of inorganic–organic polymer and exhibits high flame retardant efficiency, due to the synergy between phosphorus and nitrogen. Thermal stability, flame retardant properties and thermal degradation behavior of the composites are studied. The flame retardant mechanism based on gas and condensed actions is investigated.
2. Experimental part
2.1 Materials
PLA (a density of 1.27 g cm−3 and a mold shrinkage of 2%) was supplied by Polymer UNIC Technology Co., Ltd (Suzhou, China). EG was obtained from Qingdao Carbon Materials Co., Ltd (Qingdao, China). SPB-100 was purchased from Huizhou Sunstar Technology Co., Ltd (Huizhou, China). Its chemical structure was shown in Fig. S1.† All reagents were used as received without further purification.2.2 Preparation of flame retardant PLA composites
Before processing by melt-blending, PLA, EG and SPB-100 were dried in an oven at 80 °C for 12 hours. The resin and additives were melt blended in a twin roller mill at 180 °C, with a roller speed of 60 rpm and a mixing time of 10 minutes. The formulations of the samples were listed in Table 1. After mixing, the resultant samples were hot-pressed by a press vulcanizer at 185 °C under 5 MPa for 5 minutes, then under 10 MPa for 3 minutes to prepare sheets for further characterization.| Sample | PLA (wt%) | EG (wt%) | SPB-100 (wt%) |
|---|---|---|---|
| PLA0 | 100 | — | — |
| PLA1 | 95 | — | 5 |
| PLA2 | 90 | — | 10 |
| PLA3 | 85 | — | 15 |
| PLA4 | 85 | 15 | — |
| PLA5 | 92.5 | 3.75 | 3.75 |
| PLA6 | 90 | 5 | 5 |
| PLA7 | 85 | 7.5 | 7.5 |
| PLA8 | 85 | 5 | 10 |
| PLA9 | 85 | 10 | 5 |
| PLA10 | 85 | 11.25 | 3.75 |
| PLA11 | 85 | 3.75 | 11.25 |
2.3 Characterization
According to ASTM D2863-97 standard, LOI was measured on a HC-2 oxygen index meter (Jiangning Analysis Instrument Co., Ltd, China). The dimensions of LOI test specimens were 100 × 6.7 × 3 mm3. UL-94 vertical burning test was conducted on a CZF-II horizontal and vertical burning tester (Jiangning Analysis Instrument Co., Ltd, China) on the basic of ASTM D3801 standard. The dimensions of UL-94 vertical burning test specimens were 127 × 12.7 × 3 mm3. Thermogravimetric analysis (TGA) was conducted on a TA Q5000IR thermoanalyzer (TA Instruments Inc., USA) from room temperature to 700 °C with a heating rate of 20 °C min−1 in nitrogen and air atmosphere. Combustion property was investigated on a cone calorimeter (Fire Testing Technology, UK) on the basic of ISO 5660 standard. The dimensions of sheets were 100 × 100 × 3 mm3. Each sample was exposed to 35 kW m−2 external heat flux. A Nicolet 6700 Fourier transform infrared (FTIR) spectrophotometer (Nicolet Instrument Co., USA) was used to analyze chemical structure of char residues. The sample was mixed with KBr, and then pressed into tablet. The wavenumber range of FTIR spectrum was 4000–400 cm−1 with a 4 cm−1 resolution over 16 scans. Raman spectra of char residues were recorded on a LABRAM-HR laser confocal Raman spectroscope (Jobin Yvon Co., Ltd, France) with a 514.5 nm argon laser line. Elementary composition of char residue was investigated by X-ray photoelectron spectroscopy (XPS) on a Thermo VG ESCALAB 250 electron spectrometer (Thermo VG. Scientific Ltd, UK) with an Al Kα line (1486.6 eV) as an excitation. Thermogravimetry/Fourier transform infrared spectroscopy (TG/FTIR) and thermogravimetry/mass spectrometer (TG/MS) were employed to analyze the gaseous degradation products of PLA composites. TG/FTIR was recorded on a TGA Q5000IR thermogravimetric analyzer which was linked to a Nicolet 6700 FTIR spectrophotometer from room temperature to 700 °C with a heating rate of 20 °C min−1 in He atmosphere. TG/MS data were obtained on a Perkin-Elmer TGA analyzer coupled with a gas cell and mass spectrometer (TL-9000).3. Results and discussion
3.1 Thermal stability of PLA composites
TGA is widely applied to characterize thermal stability of materials. In this work, the temperature at 5 wt% weight loss is recognized as initial degradation temperature. Fig. 1 shows TGA and DTG curves of pure PLA, SPB-100, EG and flame retardant PLA composites under nitrogen atmosphere. The detailed TGA data are summarized in Table 2. PLA exhibits only one degradation stage, which is consistent with a single sharp peak in DTG curve. PLA starts to degrade at 339 °C with a temperature at maximum weight loss rate (Tmax) of 374 °C. SPB-100 begins to degrade at 314 °C with maximum weight loss rate occurring at 372 °C. The phosphazene flame retardant shows only one weight loss stage with a residual mass of 1.3 wt% at 700 °C. The initial degradation temperature of SPB-100 is slightly lower than that of pure PLA and higher than the processing temperature. The thermal stability of this material enables it to be a suitable flame retardant for PLA. EG also displays only one degradation stage with a char residual mass of 20.1 wt% at 700 °C and it begins to degrade at 190 °C with a Tmax of 210 °C. | ||
| Fig. 1 (a) TGA and (b) DTG curves of pure PLA, SPB-100, EG and flame retardant PLA composites under nitrogen atmosphere. | ||
| Sample | T5% (°C) | Tmax (°C) | Residue (700 °C) (wt%) |
|---|---|---|---|
| EG | 196 | 210 | 20.1 |
| SPB-100 | 314 | 372 | 1.3 |
| PLA0 | 339 | 374 | 1.8 |
| PLA3 | 335 | 377 | 2.0 |
| PLA4 | 291 | 268, 373 | 13.6 |
| PLA7 | 326 | 378 | 7.8 |
The initial degradation temperature of flame retardant PLA composites is lower than that of pure PLA, which is attributable to the thermal degradation of EG and SPB-100. However, Tmax of PLA3 and PLA7 are higher than that of pure PLA. Thermal stability of PLA7 is higher than that of PLA4, indicating that the addition of SPB-100 is beneficial to improve the thermal stability of PLA. The char yield of the composites, especially for PLA4 and PLA7, are higher than that of pure PLA. The incorporation of 15 wt% SPB-100 into PLA matrix only results in the increase of char from 1.8 to 2.0 wt%, suggesting that this flame retardant may mainly function in gaseous phase. Thus, the main component of char in PLA7 is from EG. From the DTG curves, it can be observed that the weight loss rates of composites are significantly lower than that of pure PLA.
Fig. 2 shows TGA and DTG curves of pure PLA, SPB-100, EG and flame retardant PLA composites under air atmosphere. The relevant data are summarized in Table 3. There is no marked difference between TGA curves under air and nitrogen atmosphere.
 | ||
| Fig. 2 (a) TGA and (b) DTG curves of pure PLA, SPB-100, EG and flame retardant PLA composites under air atmosphere. | ||
| Sample | T5% (°C) | Tmax (°C) | Residue (700 °C) (wt%) |
|---|---|---|---|
| EG | 199 | 212 | 21.8 |
| SPB-100 | 321 | 384 | 1.7 |
| PLA0 | 336 | 374 | 1.5 |
| PLA3 | 327 | 376 | 1.3 |
| PLA4 | 276 | 269, 365 | 9.1 |
| PLA7 | 328 | 375 | 5.9 |
The calculated TGA curves are simulated to prove synergy between SPB-100 and EG and Fig. 3 shows the calculated TGA curves of PLA7 under air and nitrogen atmosphere. The calculated TGA curves are computed by linear combination of the TGA curves of neat PLA, EG and SPB-100 according to the following equation: calculated TGA curve of PLA7 = 0.075E + 0.075S + 0.85P (where E, S and P are the TGA data of EG, SPB-100 and neat PLA, respectively.). As shown in Fig. 3, the residual mass at 700 °C in the experimental curves is markedly higher than that in the calculated curves. This is due to the presence of the synergism between SPB-100 and EG, resulting in more char residue. In condensed phase, the intumescent char layers produced by EG can work as barrier to retard the release of gaseous pyrolysis products of SPB-100. Thus, more phosphorus-containing pyrolysis products of SPB-100 are left in the condensed phase by the intumescent char layers during combustion and they combining with EG exhibit denser and larger amount of char residue.13
3.2 Flame retardant properties of PLA composites
Generally, the flammability of polymer composites is characterized by LOI and UL-94 vertical burning tests. The LOI value and UL-94 vertical burning test results are listed in Table 4. LOI value of pure PLA is only 21.0% and UL-94 vertical burning test fails (no rating). However, flame retardancy of PLA can be improved by incorporating with SPB-100 and EG. For example, both EG and SPB-100 can enhance the UL-94 rating to V-0. However, heavy dripping is observed in the composites with single component flame retardant. When 5 wt% SPB-100 is added into PLA matrix, UL-94 V-0 rating is obtained and its LOI value increases from 21.0% to 26.5%. However, higher loading of SPB-100 results in the decreased LOI value. This is explained by the fact that SPB-100 may work as a plasticizer in PLA and decrease the melt viscosity. Impressively, when 15 wt% EG is blended with PLA, UL-94 V-0 rating and a high LOI value of 39.5% are achieved. However, this composite drips heavily during the UL-94 vertical burning test. To address the problem of dripping, SPB-100 is used to combine with EG. By changing the loading and mass ratio, the best formulation is obtained, as shown in Table 4. When the mass ratio of SPB-100/EG is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the total loading of flame retardants is 15 wt%, UL-94 V-0 rating without dripping and a high LOI value of 34.5% are obtained. The excellent flame retardancy is attributed to the synergism between SPB-100 and EG. The digital photos of PLA and its composites after UL-94 vertical burning tests are shown in Fig. 4. Only PLA7 char residue remains intact without dripping.
1 and the total loading of flame retardants is 15 wt%, UL-94 V-0 rating without dripping and a high LOI value of 34.5% are obtained. The excellent flame retardancy is attributed to the synergism between SPB-100 and EG. The digital photos of PLA and its composites after UL-94 vertical burning tests are shown in Fig. 4. Only PLA7 char residue remains intact without dripping.
| Sample | LOI (%) | Dripping | Ignition of the cotton | UL-94 |
|---|---|---|---|---|
| a Y: yes; N: no; NR: no rating. | ||||
| PLA0 | 21.0 | Y | Y | NR |
| PLA1 | 26.5 | Y | N | V-0 |
| PLA2 | 25.0 | Y | N | V-0 |
| PLA3 | 24.5 | Y | N | V-0 |
| PLA4 | 39.5 | Y | N | V-0 |
| PLA5 | 25.0 | Y | Y | NR |
| PLA6 | 29.0 | Y | Y | V-2 |
| PLA7 | 34.5 | N | N | V-0 |
| PLA8 | 29.0 | Y | N | V-0 |
| PLA9 | 35.0 | Y | N | V-0 |
| PLA10 | 37.0 | Y | N | V-0 |
| PLA11 | 28.0 | Y | N | V-0 |
 | ||
| Fig. 4 Digital photos of PLA and its composites after UL-94 vertical burning tests: (a) PLA0; (b) PLA3; (c) PLA4; (d) PLA7. | ||
Cone calorimeter is a bench scale testing apparatus which is applied to characterize the combustion behavior of materials. It provides some important parameters, such as, heat release rate (HRR), total heat release rate (THR) and time to ignition (TTI). HRR curves of PLA and its composites after cone calorimeter tests are revealed in Fig. 5. The detailed information on HRR curves is listed in Table 5. It is obvious that pure PLA is susceptible to ignition and burns fast during combustion. A sharp HRR peak with a PHRR value of 410 kW m−2 is observed. With the addition of SPB-100 (PLA3), the PHRR value decreases little from 410 to 402 kW m−2. This may be due to the gaseous phase flame retardant action of SPB-100. When EG and SPB-100 are incorporated into PLA matrix (PLA7) simultaneously, the PHRR value of PLA7 decreases significantly from 410 to 196 kW m−2. Furthermore, compared with pure PLA, the THR of the flame retardant composites is markedly reduced. The heat and mass transfer is hindered by the intumescent char layers produced by EG and SPB-100 during combustion, leading to the decrease in heat release. The digital photos of char residues of PLA and its composites are showed in Fig. 6. Pure PLA is completely burned out and almost no char residue is left. A little carbon residue is observed in PLA3. Fluffy char residue is obtained in PLA4 composite. It can be seen that the char residue of PLA7 is more intact and denser than that of PLA4.
| Sample | TTI (s) | PHRR (kW m−2) | THR (MJ m−2) | Residual mass (wt%) |
|---|---|---|---|---|
| PLA0 | 12 | 410 | 27.2 | 0 |
| PLA3 | 58 | 402 | 25.7 | 0.9 |
| PLA4 | 40 | 139 | 19.8 | 8.8 |
| PLA7 | 32 | 196 | 22.6 | 6.7 |
 | ||
| Fig. 6 Digital photos of char residues of PLA and its composites after cone calorimeter tests: (a) PLA0; (b) PLA3; (c) PLA4; (d) PLA7. | ||
3.3 Flame retardant mechanism in condensed and gaseous phases
FTIR spectra of char residues of PLA0, PLA3, PLA4 and PLA7 after cone calorimeter tests are shown in Fig. S2–S4† and 7. It can be confirmed that the presence of phosphorus-containing products in the char residue of PLA7 composites. In Fig. 7, the absorption peak at 3421 cm−1 corresponds to O–H vibration adsorption of H2O. The absorption peaks at 2958, 2920 and 2848 cm−1 are assigned to the vibration adsorption of –CH3 and –CH2–, indicating the presence of some aliphatic materials in the char residue. The absorption peak at 1626 cm−1 corresponds to the vibration adsorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in graphite material.14 The peak at 1255 cm−1 may be attributed to the vibration of P
C in graphite material.14 The peak at 1255 cm−1 may be attributed to the vibration of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.15 The peaks at 1169 and 1020 cm−1 can be ascribed to the stretching vibration of PO2/PO3 in phosphate carbon complexes.16,17 The peaks at 1088 and 885 cm−1 belong to stretching vibrations of P–O–P bond. This result indicates the formation of poly(phosphoric acid), such as, P2O5 and P4O10, in which phosphate groups link to each other by sharing one oxygen atom.8 In Fig. S3,† phosphorus based compound has also been found in the char of PLA3. In summary, pyrolysis products of SPB-100 may also exhibit condensed phase flame retardant mechanism.
O.15 The peaks at 1169 and 1020 cm−1 can be ascribed to the stretching vibration of PO2/PO3 in phosphate carbon complexes.16,17 The peaks at 1088 and 885 cm−1 belong to stretching vibrations of P–O–P bond. This result indicates the formation of poly(phosphoric acid), such as, P2O5 and P4O10, in which phosphate groups link to each other by sharing one oxygen atom.8 In Fig. S3,† phosphorus based compound has also been found in the char of PLA3. In summary, pyrolysis products of SPB-100 may also exhibit condensed phase flame retardant mechanism.
XPS is used to investigate the element composition and chemical bond form. XPS wide scanning spectrum and C1s, P2p and O1s spectra of char residue of PLA7 after cone calorimeter test are shown in Fig. 8 and the relevant XPS data are listed in Table 6. In Fig. 8(a), it can be confirmed that C (70.9 at%), O (23.5 at%), P (2.1 at%), N (3.4 at%) and S (0.1 at%) are left in the char residue of PLA7. In the C1s spectrum, the peak at 284.6 eV belongs to C–H and C–C in aliphatic and aromatic species.18 The peak at 285.5 eV is assigned to C–OH, C–O–P, or C–N.19 The band at 288.3 eV is ascribed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.20 In Fig. 8(c), the peak at 134.3 eV is assigned to P–O–C or P
O.20 In Fig. 8(c), the peak at 134.3 eV is assigned to P–O–C or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.21 As shown in Fig. 8(d), the peak at binding energy of 531.1 eV corresponds to P
O groups.21 As shown in Fig. 8(d), the peak at binding energy of 531.1 eV corresponds to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in phosphate or C
O in phosphate or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.18 The peak at 532.8 eV is assigned to C–O–C, C–O–P or C–OH.22,23 In conclusion, the char residue of PLA is composed of expanded graphite and phosphorus-containing crosslinking materials.
O groups.18 The peak at 532.8 eV is assigned to C–O–C, C–O–P or C–OH.22,23 In conclusion, the char residue of PLA is composed of expanded graphite and phosphorus-containing crosslinking materials.
 | ||
| Fig. 8 (a) XPS wide scanning spectrum; (b) C1s, (c) P2p and (d) O1s spectra of char residue of PLA7 after cone calorimeter test. | ||
| Element | Binding energy (eV) | Atomic percent (at%) |
|---|---|---|
| C1s | 284.6, 285.5, 288.3 | 70.9 |
| O1s | 531.1, 532.8 | 23.5 |
| N1s | 400.2 | 3.4 |
| P2p | 134.3 | 2.1 |
| S2p | 169.0 | 0.1 |
Raman spectroscopy is used to characterize the graphitization degree of char residues. Fig. 9 shows Raman spectra of char residues of PLA3, PLA4 and PLA7. The peak at around 1593 cm−1 corresponds to G band which is caused by the vibration of sp2-hybridized carbon atoms in graphite layer. Besides, G band is corresponding to an E2g mode of hexagonal graphite.24,25 The peak at around 1350 cm−1 is D band which is attributed to the vibration of carbon atoms with dangling bonds in the plane terminations of disordered graphite or glassy carbons.24,25 The char residue of PLA4 shows very high graphitization degree, due to the incorporation of EG. The graphitization degree of PLA3 (ID/IG = 3.50) is lower compared with others, indicating the formation of amorphous carbon from SPB-100. The graphitization degree of the char residue of PLA7 is higher than that of PLA3, but lower than that of PLA4. It also suggests that the char residue of PLA7 consists of graphitic and amorphous carbon. The char residue containing amorphous carbon and phosphorus-containing crosslinking products may act as adhesive to expanded graphite, leading to denser char residue of PLA7.
To investigate thermal decomposition of PLA and its composites, TGA/FTIR is employed to detect the gaseous products of PLA and PLA7 during the thermal degradation. The primary pyrolysis products of pure PLA are CO2, CO, acetaldehyde, methane and butanedione.26–28 Fig. 10 shows FTIR spectra of gaseous products of PLA0 and PLA7 at maximum decomposition rate. H2O (3578 cm−1), CO2 (2358 cm−1), CO (2182 cm−1), hydrocarbons (–CH3 and –CH2– groups: 3002–2850 and 1400–1200 cm−1), aldehyde (2740 cm−1) and carbonyl group (1762 cm−1) are observed in the gaseous products of PLA0.19,29,30 Actually, the main FTIR signals of PLA7 are similar to those of pure PLA. From Fig. 10(b), the absorption peaks for P–O–C (1102 cm−1) and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1257 cm−1) are observed, indicating the presence of phosphorus-containing fragments in the gas pyrolysis products of PLA7.31
O (1257 cm−1) are observed, indicating the presence of phosphorus-containing fragments in the gas pyrolysis products of PLA7.31
The absorbance of pyrolysis products of pure PLA and PLA7 versus time is revealed in Fig. 11. It is obvious that pure PLA and PLA7 start to release pyrolysis products at almost the same time. The intensity of total pyrolysis products, as well as H2O, CO2, CO, hydrocarbons and carbonyl compounds, of PLA7 is lower than those of pure PLA. This can be illuminated by the fact that the high quality intumescent char produced by EG and SPB-100 can act as a physical barrier and hinder the volatilization of degradation products of PLA.
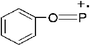
TG/MS is used to investigate the possible gas phase flame retardant mechanism of SPB-100. The total ion current (TIC) chromatograms from TG/MS are presented in Fig. 12. The mass spectra corresponding to the TIC peaks of neat PLA and PLA7 are shown in Fig. 13. The m/z peaks at 55, 56, 71 and 73 belong to C3H3O, C3H4O, C3H3O2 and C3H5O2, respectively.28 The m/z peaks from 184 to 252 are ascribed to the cyclic and acyclic oligomers from the pyrolysis of pure PLA.28 Compared with Fig. 13(a), there is an obvious difference in Fig. 13(b) with respect to the characteristic fragment ionic peaks. The detailed structure of characteristic fragment ionic peaks of PLA7 is presented in Table 7. For example, the m/z peaks at 63, 65, 93 and 124 are corresponding to PO2, C5H5, C6H5O and C6H5OP, respectively, due to the pyrolysis of SPB-100.13 It is well acknowledged that the free radicals, such as PO2˙, C6H5O˙ and C6H5OP˙, can retard the combustion reaction of polymer in gas phase.13 Thus, this phosphazene polymer is expected to function by quenching the flammable free radicals, such as H˙ and HO˙.
Flame retardant mechanism of PLA/EG/SPB-100 composite is illustrated in Fig. 14. In condensed phase, intumescent char layers produced by EG can work as physical barrier to inhibit the heat and mass transfer and pyrolysis of polymer. At the same time, due to hindrance of the intumescent char layers, more phosphorus-containing pyrolysis products of SPB-100 are left in condensed phase resulting in denser and larger amount of char residue. In gaseous phase, the flame-quenching free radicals from the pyrolysis of SPB-100 show the gaseous phase flame retardant mechanism. In summary, this flame retardant system exhibits the condensed and gaseous phase flame retardant mechanism in PLA simultaneously.
4. Conclusions
In this work, flame retardant PLA composites were prepared by melt blending approach. UL-94 V-0 rating, high LOI value and anti-dripping performance were obtained in PLA composite with low loading of EG and poly(bis(phenoxy)phosphazene). The heat release of the PLA composite during the combustion was greatly reduced. The analysis of char residue was conducted by FTIR, Raman spectroscopy and XPS. The gas pyrolysis products were analyzed by TG/FTIR and TG/MS. The synergy between SPB-100 and EG was proved. The excellent flame retardancy of PLA composite was attributed to the combined action of gas and condensed flame retardant mechanism of EG and SPB-100.Acknowledgements
The authors acknowledge the research grants from the National Basic Research Program of China (973 Program) (Grant No. 2014CB931804), the National Natural Science Foundation of China (Grant No. 51323010) and the Fundamental Research Funds for the Central Universities (Grant No. WK2320000032).References
- W. S. Chow and E. L. Teoh, J. Appl. Polym. Sci., 2015, 132, 41253 CrossRef PubMed.
- F. Liao, L. Zhou, Y. Ju, Y. Yang and X. Wang, Ind. Eng. Chem. Res., 2014, 53, 10015 CrossRef CAS.
- K. Tao, J. Li, L. Xu, X. Zhao, L. Xue, X. Fan and Q. Yan, Polym. Degrad. Stab., 2011, 96, 1248 CrossRef CAS PubMed.
- M. Murariu, L. Bonnaud, P. Yoann, G. Fontaine, S. Bourbigot and P. Dubois, Polym. Degrad. Stab., 2010, 95, 374 CrossRef CAS PubMed.
- M. Shabanian, N.-J. Kang, D.-Y. Wang, U. Wagenknecht and G. Heinrich, Polym. Degrad. Stab., 2013, 98, 1036 CrossRef CAS PubMed.
- K. Kimura and Y. Horikoshi, Fujitsu Sci. Tech. J., 2005, 41, 173 CAS.
- C.-H. Ke, J. Li, K.-Y. Fang, Q.-L. Zhu, J. Zhu, Q. Yan and Y.-Z. Wang, Polym. Degrad. Stab., 2010, 95, 763 CrossRef CAS PubMed.
- J. Zhan, L. Song, S. Nie and Y. Hu, Polym. Degrad. Stab., 2009, 94, 291 CrossRef CAS PubMed.
- G. Tang, X. Wang, R. Zhang, B. Wang, N. Hong, Y. Hu, L. Song and X. Gong, Ind. Eng. Chem. Res., 2013, 52, 7362 CrossRef CAS.
- P. Wei, S. Bocchini and G. Camino, Polimery, 2013, 58, 361 CrossRef CAS.
- X. Zhou, J. Li and Y. Wu, Polym. Adv. Technol., 2015, 26, 255 CrossRef CAS PubMed.
- Z. Yang, Z. Wang, J. Li and J. Chen, RSC Adv., 2012, 2, 11432 RSC.
- L. Qian, F. Feng and S. Tang, Polymer, 2014, 55, 95 CrossRef CAS PubMed.
- Y. Bihe, B. Chenlu, Q. Xiaodong, S. Lei, T. Qilong, L. Kim Meow and H. Yuan, Carbon, 2014, 75, 178 CrossRef PubMed.
- D. L. Wang, Y. Liu, D. Y. Wang, C. X. Zhao, Y. R. Mou and Y. Z. Wang, Polym. Degrad. Stab., 2007, 92, 1555 CrossRef CAS PubMed.
- W. Liu, D.-Q. Chen, Y.-Z. Wang, D.-Y. Wang and M.-H. Qu, Polym. Degrad. Stab., 2007, 92, 1046 CrossRef CAS PubMed.
- M. Bugajny, S. Bourbigot, M. Le Bras and R. Delobel, Polym. Int., 1999, 48, 264 CrossRef CAS.
- Y. Bihe, B. Chenlu, S. Lei, H. Ningning, L. Kim Meow and H. Yuan, Chem. Eng. J., 2014, 237, 411 CrossRef PubMed.
- K. Wu, Y. Hu, L. Song, H. Lu and Z. Wang, Ind. Eng. Chem. Res., 2009, 48, 3150 CrossRef CAS.
- X. Wang, L. Song, H. Yang, H. Lu and Y. Hu, Ind. Eng. Chem. Res., 2011, 50, 5376 CrossRef CAS.
- W. Chen, P. Liu, Y. Liu and Q. Wang, Polym. Chem., 2015, 6, 4409 RSC.
- X. Chen, Y. Hu, C. Jiao and L. Song, Polym. Degrad. Stab., 2007, 92, 1141 CrossRef CAS PubMed.
- P. Shih, S. Yung and T. Chin, J. Non-Cryst. Solids, 1998, 224, 143 CrossRef CAS.
- A. Rao, E. Richter, S. Bandow, B. Chase, P. Eklund, K. Williams, S. Fang, K. Subbaswamy, M. Menon and A. Thess, Science, 1997, 275, 187 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS PubMed.
- C.-A. Nicolae, M. A. Grigorescu and R. A. Gabor, Eng. Lett., 2008, 16, 568 Search PubMed.
- O. Wachsen, K. Reichert, R. Krüger, H. Much and G. Schulz, Polym. Degrad. Stab., 1997, 55, 225 CrossRef CAS.
- F.-D. Kopinke, M. Remmler, K. Mackenzie, M. Möder and O. Wachsen, Polym. Degrad. Stab., 1996, 53, 329 CrossRef CAS.
- L. Hai-Juan, H. Li-Jing, W. Xue-Mei, B. Yi-Jie, L. Yue-Sheng and D. Li-Song, Polym. Adv. Technol., 2013, 24, 576 CrossRef PubMed.
- U. Braun, A. I. Balabanovich, B. Schartel, U. Knoll, J. Artner, M. Ciesielski, M. Döring, R. Perez, J. K. Sandler and V. Altstädt, Polymer, 2006, 47, 8495 CrossRef CAS PubMed.
- L. Song, S. Xuan, X. Wang and Y. Hu, Thermochim. Acta, 2012, 527, 1 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra12701g |
| This journal is © The Royal Society of Chemistry 2015 |