Effect of using sonicated sulphuric acid as an electrolyte in a lead acid battery†
Mithin kumar S.,
Sundar Mayavan*,
Ganesan M. and
S. Ambalavanan*
CSIR-Central Electrochemical Research Institute, Karaikudi, India. E-mail: vananambu456@yahoo.co.in; sundarmayavan@cecri.res.in
First published on 2nd September 2015
Abstract
The consequences of adding sonicated sulphuric acid as an electrolyte in a lead acid battery (LAB) have been investigated. The LAB fabricated using sonicated sulphuric acid electrolyte shows improved performance (electrolyte conductivity and capacity) than LAB with normal sulphuric acid electrolyte.
The lead acid battery (LAB) is one of the most successful and oldest rechargeable electrochemical systems in existence. Even 150 years after its discovery, there is no actual replacement or alternative for this technology. The main reasons being low cost, ease of manufacture, high durability, high safety and full recyclability. During the last decade, the major part of LAB research has been focussed in the improvement of its performance like energy density, cyclability, etc.1–8 Various strategies have been proposed to improve the performance of LAB. Most of the reported work focuses on improving the performance of LAB via modification of active components like positive active material (PAM) and negative active material (NAM). Whereas much less attention was paid to electrolyte. In LAB, the electrolyte sulphuric acid plays a vital role as an active material which takes part in electrochemical reactions during cycling of lead acid batteries. It is well known that transport properties of electrolyte/solution can be improved via activation. Among various methods (for activation), sonication is considered to be the simplest, which leads to improvement and enhancement of ionic mass transfer in variety of solution. Ultrasound is known for increasing mass transfer in electrochemical process and also increases the quantity of free ions in the electrolyte, thereby its conductivity.9,10 Donald A. Dornbusch et al., studied sonication as a technique to reduce losses associated with diffusion for zinc–manganese oxide alkaline cells.11 M. L. Doche et al., studied the corrosion passivation mechanism of zinc in sonicated sodium hydroxide solutions.12
In LAB, the reaction depends on the diffusion and migration of ions between the electrodes, which needs to be improved to achieve enhancement in capacity. In this paper we report for the first time the effect of using sonicated sulphuric acid as an electrolyte in LAB. The LAB fabricated using sonicated sulphuric acid electrolyte shows improved performance (electrolyte conductivity and capacity) than LAB with normal sulphuric acid electrolyte.
Lead acid test cell (2 V/1.88 A h) was assembled comprising one negative and two positive plates using lead–selenium alloy grids. Dimensions of negative and positive plates were 48 mm × 39 mm × 1.8 mm and 48 mm × 39 mm × 1.8 mm respectively. The negative lead paste was prepared by conventional method. In the conventional method, negative active material was prepared by mixing grey oxide (73% PbO + 27% free Pb), carbon black (0.25%), lignin (0.3%), and barium sulfate (0.3%) in a sigma mixture. Dry mixing was carried out for 2–3 minutes. Then demineralized water was added as quickly as possible into dry mix. Then H2SO4 (sp.gr 1.3) was added as slowly as possible in order to prevent the paste temperature going above 60 °C. Finally small portion of water was added (during mixing) to bring the paste to proper consistency. The finished paste was applied to the grid. Then the plates are cured under carefully controlled conditions of time (48 hour), temperature (<60 °C) and relative humidity (>90%). Then dried plates are electrolytically oxidised and reduced in dilute H2SO4 (sp.gr 1.05) solution. Finally the cells were assembled with PVC separator inserted between positive and negative plates. The cells were filled with 40 ml of 1.26 sp.gr of sonicated sulphuric acid (H2SO4) prepared via bath sonication using Rivotek 40 Hz sonicator for 30 minutes. As a control one test cell filled with unsonicated 1.26 sp.gr sulphuric acids were also assembled. The charge–discharge tests were carried out using Bitrode life cycle tester. Electrochemical impedance spectroscopy (EIS) measurements were carried out using AUTOLAB PGSTAT 30 in the frequency range 100 kHz to 10 mHz with an AC voltage of 5 mV. Cyclic voltammetry (CV) studies were carried out using 0.5 cm2 lead foil in the potential range −1.0 V to +2.0 V with a scan rate of 5 mV s−1. The potential measured were with respect to Hg/Hg2SO4 (1.26 sp.gr H2SO4). In order to confirm the increased reactivity of sonicated electrolyte and also to infer the action of sonication on sulphuric acid electrolyte, conductivity measurements were made on sulphuric acid electrolyte with and without sonication. Specific gravity of sulphuric acid is 1.260. Sulphuric acid is subjected to sonication for 30 minutes. Immediately after sonication and with respect to time (in days), conductivity measurements were carried out and tabulated in Table 1. The conductivity of sonicated sulphuric acid was found to be higher. But the conductivity of acid without sonication is almost constant. Sonication may induce several phenomena like acoustic streaming, acoustic cavitation, microjet formation that could result in increase of free ions in the electrolyte, but what is more interesting is that this effect gets maintained even after sonication. It is to be noted that the conductivity value was monitored for 20 days and no significant change in conductivity value was observed after 9th day.
| Time (days) | 0 | 1 | 3 | 4 | 6 | 9 |
| Acid sonication | 772.8 | 773.9 | 776.8 | 778.0 | 787.5 | 780.2 |
| Unsonicated acid | 753.0 | 753.5 | 754.4 | 754.8 | 753.5 | 753.7 |
Fig. 1 shows the cyclic voltammogram (CV) of lead foil in sonicated and unsonicated sulphuric acid (electrolyte). Fig. 1 shows pronounced anodic peak (a1) (at −0.61 V) and a cathodic peak: at −0.68 V (peak c1), followed by a passive region. The peak a1 and c1 corresponds to oxidation of Pb to PbSO4 and reduction of formed PbSO4, respectively. Above +2.0 V oxygen evolution takes place and the current increases sharply which is not indicated in the CV. It is interesting to note that peak current obtained with sonicated sulphuric acid solution is much higher than those obtained with unsonicated solution. This clearly indicates that anodic and cathodic processes are more intensified upon sonication and gets maintained even after 25 potential cycles. This is a clear indication that effect of sonication gets maintained even after repeated redox cycles. To further understand the effect (of sonicated acid), we fabricated LAB using the sonicated electrolyte and its performance (the capacity) was compared with LAB fabricated with normal unsonicated sulphuric acid electrolyte. The cell was assembled with 1.88 A h capacity as per standard method. Fig. 2 shows the comparison between the performance of sonicated and conventional cell. Fig. 2a shows the comparison of obtained capacity at different discharge rate with respect to cycles. Under all discharge rates, the discharge capacity of cell assembled with sonicated electrolyte (sonicated cell) was much higher than those of unsonicated acid added cell. Table 2 shows the improvement in capacity and percentage of utilization of the active material derived from Fig. 2a. The obtained utilization of active material in terms of percentage is 1.78, 3.57, 6.44, and 5.29 for different rates such as C/20, C/10 and C/5 respectively at 100% depth of discharge. The increase in the utilization of active material as the discharge rate goes up clearly indicates the increased availability of ions in the sonicated electrolyte which in turn indicates the increase in the mobility of ions which penetrates inside the oxide electrode at higher discharge rate. Fig. 2b shows the comparison of charge discharge at C/20 rate for cells with sonicated and without sonicated electrolyte. Fig. 3 shows the Nyquist plot for cell filled with sonicated and unsonicated acid at full charge condition. The solution transfer resistance for sonicated cell is lower than that of conventional cell. The improvement in the discharge time for sonicated electrolyte clearly reveals the importance of using sonicated electrolyte in the cell in enhancing the capacity of the cell. From Fig. 2, it is evident that sonicated electrolyte shows marked improvement in the capacity of the cell as compared to unsonicated cell. As a control, we have performed the experiments with commercial 12 V/5 A h battery with and without sonicated sulphuric acid. It has been observed that LAB filled with sonicated sulphuric acid showed significant increase in capacity as compared to commercial LAB filled with unsonicated acid (Fig. S1†). These results are in consistence with the above data (Fig. 2a).
 | ||
| Fig. 2 (a) Comparison between sonicated and conventional cell capacity at different rates of discharge (b) charge–discharge curve for sonicated and without sonicated electrolyte at C/20 rate. | ||
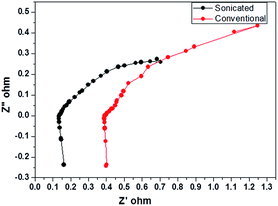 | ||
| Fig. 3 Nyquist plot for cell filled with sonicated electrolyte and conventional electrolyte at full charge condition. | ||
Sonication is an acoustic energy or a sound wave which involves the conversion of an electrical signal into a physical vibration. During sonication, the air bubble collapses which leads to microjet formation, and the local temperature, pressure, and velocity can reach as high as 5000 K, 6 kbar and 4 km s−1 respectively. Under such a condition, mass transport will be enhanced and complete ionisation of sulphuric acid and high concentration of free ions is possible, which is impossible under normal conditions.9 The availability of high concentration of free ions leads to high utilization and hence higher capacity as evidenced by Fig. 2. Further the cell is under charging/discharging continuously which means continuous electrolysis of sonicated electrolyte is going on during lead acid cell operation. Hence after each cycle the amount of free ions generated by sonication will be maintained (than with unsonicated H2SO4 electrolyte) and hence higher utilization and higher capacity.
In conclusion, simple use of sonicated electrolyte in the lead acid cell show marked improvement in the utilization of active material which gives rise to enhanced capacity. Capacity has been evaluated for C/20, C/10, C/5 and C/2 rates. The enhancement in capacity of the lead acid cell is substantiated by increased availability of free ions leading to increased conductivity of the electrolyte and reactivity with electrode material. This approach can be easily adopted in existing battery manufacturing industry since in this study the whole cell is not subjected to sonication and only previously sonicated electrolyte is used which results in improved performance.
Acknowledgements
This work was supported by Council of Scientific and Industrial Research (CSIR), India – TAPSUN project NWP 56A.Notes and references
- D. R. Battle bury, J. Power Sources, 1999, 80, 7 CrossRef CAS.
- I. Petersson and E. Ahlberg, J. Power Sources, 2000, 91, 143 CrossRef CAS.
- A. Czerwinski, S. Obrebowskia, J. Kotowski, Z. Rosalski, J. M. Snowronskic, P. Krawczyk, T. Rozmanowski, M. Bajsert, M. Przystalowski, M. Buczkowska biniecka, E. Jankowska and M. Baranick, J. Power Sources, 2010, 105, 7530 CrossRef PubMed.
- A. Kirchev, N. kircheva and M. ferrin, J. Power Sources, 2011, 196, 8773 CrossRef CAS PubMed.
- S. K. Martha, B. Hariprakash, S. A. Gaffoor, D. C. Trivedi and A. K. Shukla, J. Chem. Sci., 2006, 118, 93 CrossRef CAS.
- R. shapira, G. D. Nessim, T. Zimrin and D. Aurbach, Energy Environ. Sci., 2013, 6, 587 CAS.
- M. Saravanan, P. Sennu, M. Ganesan and S. Ambalavanan, J. Electrochem. Soc., 2013, 160, A70 CrossRef CAS PubMed.
- D. Pavlov, P. Nikolov and T. Rogachev, J. Power Sources, 2010, 195, 4444 CrossRef CAS PubMed.
- F. Marken, R. G. Compton, S. G. Davies, S. D. Bull, T. Thiemann, M. Luisa Sá e Melo, A. Campos Neves, J. Castillo, C. G. Jung and A. Fontana, J. Chem. Soc., Perkin Trans. 2, 1997, 2055 RSC.
- R. G. Compton, F. Marken and T. O. Rebbitt, J. Chem. Soc., Chem. Commun., 1996, 1017 RSC.
- D. A. Dornbusch, R. Hilton, M. J. Gordon and G. J. Suppes, ECS Electrochemistry Letters, 2013, 2, 89 CrossRef PubMed.
- M.-L. Doche, J.-Y. Hihn, F. Touyeras, J. P. Lorimer, T. J. Mason and M. Plattes, Ultrason. Sonochem., 2001, 8, 291 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Comparison between sonicated and conventional lead acid battery (12 V/5 A h; make: AMCO) capacity at different rates of discharge. See DOI: 10.1039/c5ra17286a |
| This journal is © The Royal Society of Chemistry 2015 |

