High-yielding and facile synthesis of organosilicon compounds containing a m-carboranylmethyl group†
Abstract
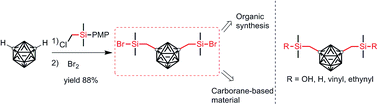
An efficient method for the synthesis of organosilicon compounds containing m-carboranylmethyl was developed, which afforded the products in good to excellent yields (up to 88%) compared to the literature methods affording a 38% yield. Moreover, the generated intermediate 5 containing a Si–Br bond could be functionalized conveniently.

 Please wait while we load your content...
Please wait while we load your content...