Selective esterification to produce microalgal biodiesel and enrich polyunsaturated fatty acid using zeolite as a catalyst
Tao Dong*a,
Xiaochen Yua,
Chao Miaoa,
Barbara Rascob,
Manuel Garcia-Péreza,
Shyam S. Sablania and
Shulin Chen*a
aDepartment of Biological Systems Engineering, Washington State University, Pullman, WA 99164, USA. E-mail: tao_dong@wsu.edu; chens@wsu.edu; Fax: +1-509-335-2722; Tel: +1-509-335-3743
bSchool of Food Science, Washington State University, Pullman, WA 99164, USA
First published on 30th September 2015
Abstract
Microalgae can be both a promising biofuel feedstock and a source of polyunsaturated fatty acids (PUFA). This paper reports a novel integrated process that simultaneously produces biodiesel and enriches PUFA. It was accomplished by using zeolite as a selective catalyst that preferentially converts shorter-chain fatty acids (SCFA) into fatty acid methyl esters (FAME) (86% conversion for S. limacinum and 65% conversion for N. salina) and enriches high-value PUFA (70% for S. limacinum and 78% for N. salina) in the unreacted free fatty acid (FFA) stream. The esterification reaction rate was affected by acid strength and pore size, while the selectivity of zeolite increased as pore size of zeolite decreased. This approach allows production of high quality biodiesel and efficient PUFA enrichment. The unreacted PUFA can be further refined for nutraceutical or other applications to improve economic viability of microalgal biodiesel production.
1. Introduction
Microalgae have been considered as a promising source of biofuel feedstock due to their high lipid productivity compared to land crops.1,2 However, the cost of microalgal lipid is still very high for the intended fuel applications. Development of algal biorefinery options with a diverse slate of value-added coproducts is required to make algal biofuel commercially possible.Besides C14–C18 fatty acids as the main fractions of lipid, some algae may also contain considerable amount of high-value longer-chain omega-3 polyunsaturated fatty acid (PUFAs) such as arachidonic acid (ARA, C20:4), eicosapentaenoic acid (EPA, C20:5), docosapentaenoic acid (DPA, C22:5) and docosahexaenoic acid (DHA, C22:6).3–6 For example, the autotrophic Nannochloropsis sp. and heterotrophic Schizochytrium limacinum are both promising for biofuel productions due to their high lipid contents.7 S. limacinum is a strain identified as being capable of utilizing glycerol as a carbon source to produce DHA.4 Nannochloropsis salina is a marine species that are considered as an important sources of EPA and widely used in marine aquaculture nutrition.5 EPA and DHA are well known dietary essential fatty acids and have well-known health benefits.8,9 Currently, microalgal oil is the primary source of DHA for infant formula use.10 In addition, PUFAs in general are promising precursors for producing biodegradable and nontoxic biopolymer building blocks via epoxidation. Fatty acid based epoxides and polyols are important starting materials for making polyurethanes and epoxy resins with similar characteristics to petrochemical polyurethanes, and have been produced from crude algal oils.11 The efficiency of these epoxides is directly related to the amount of epoxy groups per molecule, expressed with an oxirane number. Epoxides derived from PUFA with higher oxirane values and lower iodine values is expected to be high-quality plasticizers.12
Fractionating PUFAs from microalgal lipid feedstock adds value to microalgal biofuel production, as well as improving the quality of microalgae-based biodiesel. This is because PUFAs are prone to oxidation and have poor thermal stability, which leads to coke formation in engines.13 PUFAs are conventionally separated and purified in the food industry using various methods, such as formation of a urea complex, chromatographic separation, selective solvent extraction, salt precipitation and enzymatic splitting.14 However, these methods are inefficient; require expensive equipment or use environmentally-undesirable solvents. A cost-effective and scalable approach is needed to recover PUFAs as co-products from microalgal oils to facilitate use of these feedstocks in biofuel production.
Zeolites are popular industrial catalysts comprised of crystalline microporous materials of different molecular sizes and shapes. They are widely used in oil refining, petrochemical production, and organic synthesis of important chemicals. The intricate channel structure of zeolites provides selectivity based upon shape and polarity, and is particularly effective for reactant molecules with a kinetic diameter below 10 Å.15
This study explored the potential of selectively converting low-value, short-chain fatty acids (SCFA, C12–C18) in microalgae oil into fatty acid methyl esters (FAME), while retaining high-value, long-chain fatty acids (LCFA) in their original free fatty acid (FFA) form through selective esterification. Unreacted PUFA can differentiate from esterified SCFA in the chemical structure, possibly facilitating more effective downstream separation (Scheme 1).
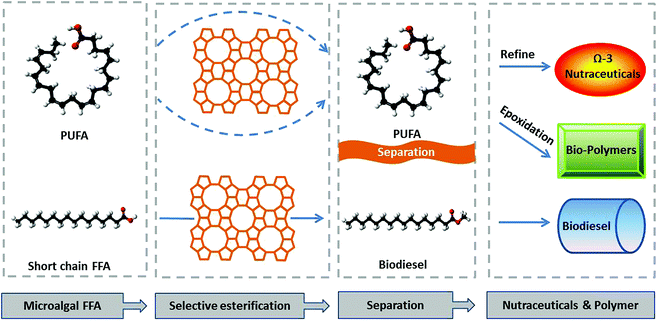 | ||
| Scheme 1 Selective esterification to produce biodiesel and fractionate PUFA for value-added bioproducts. | ||
2. Experimental
2.1 Materials
Zeolite HZSM-5 (SiO2/Al2O3 = 90 and H+ balancing cations) was supplied by Sud-Chemie (Louisville, KY). Zeolite Hβ CP811E-150 (SiO2/Al2O3 = 75) and zeolite HY CBV-600 (SiO2/Al2O3 = 5.2) were supplied by Zeolyst International (Conshohocken, PA).Schizochytrium limacinum SR21 (ATCC MYA-1381) was cultured in artificial seawater for DHA production.4 Nannochloropsis salina was supplied by New Mexico State University (Las Cruces, NM, US). Microalgae were harvested by centrifugation and lyophilized before use.
Tridecanoic acid methyl ester, docosapentaenoic acid methyl ester and Supelco 37 FAME mix were purchased from Sigma Chemical Co. (St. Louis, MO, US). All solvents and reagents were either of HPLC grade or analytical reagent grade.
2.2 Microalgal oil extraction and processing
The lyophilized microalgae powder (N. salina and S. limacinum) were extracted by hexane in Soxhlet apparatus for 12 h, respectively. The organic solvent was vacuum-evaporated to obtain the microalgal oil. Chlorophyll in N. salina oil was removed by adding 1% (v/v) of phosphoric acid and heated at 100 °C under a vacuum for 30 min.16 The oil was separated from the dark green chlorophyll floccules, resulting in a refined N. salina oil. Since there is no chlorophyll in S. limacinum, no chlorophyll removal process was needed for this oil. The refined N. salina oil and S. limacinum oil were refluxed with 1 M KOH in 95% ethanol (v/v) for 1 hour saponification, respectively. Equal volume of hexane was used to extract unsaponificated compounds. Then, the hydrolysis solution was acidified with sulfuric acid, and free fatty acids (FFA) were extracted with hexane. The hexane solvent was removed by vacuum evaporation and the obtained FFA was washed with an equal volume of water 3 times before selective esterification.172.3 Esterification of FFA
FAME was prepared via a rapid derivatization procedure. An aliquot (20 mg) of each prepared FFA sample (from S. limacinum and N. salina), 1 mL of tridecanoic acid (0.5 mg mL−1), 5 mL of methanol and 0.5 mL of 14 M KOH water solution were added into the Pyrex screw-cap tube (16 × 125 mm). The tube was vortexed for 10 s before being placed in an 85 °C water bath for 15 min for alkaline hydrolysis. After the alkaline hydrolysis, the tube was cooled down with tap water to room temperature. Then, 0.58 mL of 12 M H2SO4–methanol solution was added. The tube was vigorously agitated for 1 min, and then incubated in an 85 °C water bath for 15 min to for acidic esterification. Afterwards, the tube was cooled with tap water and 2 mL of hexane and 2 mL of water were added. FAME extraction was carried out by 2 min of vortex on a Tekmar VXR-10 multitube vortex. The tube containing the FAME extract was centrifuged at 1500g for 2 min and 1 μL of hexane phase was injected for GC analysis.62.4 Selective esterification of FFA
The FFA and methanol was fed into a Pyrex tube reactor with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Zeolite (10 wt% based on FFA) was added to catalyze the esterification. The reaction was performed in a silicon oil bath at 85 °C with a stirring rate of 150 rpm. Samples were taken from the reaction mixture at different time intervals for GC analysis.
30. Zeolite (10 wt% based on FFA) was added to catalyze the esterification. The reaction was performed in a silicon oil bath at 85 °C with a stirring rate of 150 rpm. Samples were taken from the reaction mixture at different time intervals for GC analysis.
The FAME conversion, FFA recovery and FFA enriched ratio of individual fatty acid were calculated with the following equations.
 | (1) |
 | (2) |
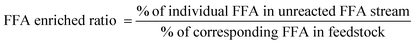 | (3) |
2.5 GC analysis
FAME analysis was performed on an Agilent 7890A GC equipped with an auto-sampler (Agilent 7683B), a flame ionization detector (FID) and a FAMEWAX column (30 m × 320 μm × 0.25 μm) (Restek, Bellefonte, PA). Helium was used as the carrier gas. The injection volume was 1 μL with split ratio at 20. The parameters of the oven temperature program started at 40 °C with 3 °C min−1 intervals up to 250 °C and held for 20 min. The temperatures of the injector and detector were set at 300 °C and 250 °C, respectively. Tridecanoic acid methyl ester was used as the internal standard and added before GC analysis.63. Results and discussion
3.1 Fatty acid composition of FFA feedstock
As shown in Table 1, S. limacinum oil is rich in DPA and DHA, while N. salina is rich in EPA and ARA. This result agrees with previous reports.5,18 The DPA, DHA, EPA and ARA are all high-value PUFA, which are used in food supplements and nutraceuticals.19 In terms of biodiesel production, fractionation of PUFA is also desirable for high-quality bio-diesels with improved oxidation stability20 and combustion performance.21| Fatty acid | Fatty acid content (%) | ||
|---|---|---|---|
| S. limacinum | N. salina | ||
| a Only the most abundant fatty acids are shown. | |||
| Myristic acid | C14:0 | 5.28 ± 0.07 | 3.93 ± 0.08 |
| Pentadecanoic acid | C15:0 | 1.42 ± 0.04 | — |
| Palmitic acid | C16:0 | 57.75 ± 0.11 | 45.48 ± 0.19 |
| Hexadecenoic acid | C16:1 | — | 28.65 ± 0.06 |
| Oleic acid | C18:1 | 2.01 ± 0.05 | 13.01 ± 0.21 |
| Arachidonic acid | C20:4 | — | 2.71 ± 0.01 |
| Eicosapentaenoic acid | C20:5 | — | 6.23 ± 0.06 |
| Docosapentaenoic acid | C22:5 | 7.51 ± 0.02 | — |
| Docosahexaenoic acid | C22:6 | 26.04 ± 0.04 | — |
3.2 Physico-chemical properties of zeolite catalysts
The physico-chemical properties of zeolites catalysts are shown in Table 2. Zeolites used in this study have increasing pore size following the order of HZSM-5 < Hβ < HY, indicating a better selectivity of HZSM-5 to FFA molecular with a smaller kinetic diameter. The constraint index (CI) is commonly used to investigate the shape selectivity of zeolites.22 It is defined as the ratio of the observed cracking rate constants of n-hexane to 3-methylpentane. A higher CI value indicates a larger steric hindrance, while a lower CI value indicates the absence of steric hindrance. As shown in Table 2, the steric hindrance increased following the order of HY < Hβ < HZSM-5.3.3 The total esterification conversion
All three zeolites tested in this study could catalyze esterification of FFA (Fig. 1). The total conversion rate of FFA into FAME followed the order of Hβ > HZSM-5 > HY. The esterification rate is believed to be determined by the acidity strength and pore size of the catalysts. Generally, the acidic strength is determined by the zeolite crystal structure as follows: HZSM-5 (MFI) > Hβ (BEA) > HY (FAU).27 The higher esterification rate of Hβ and HZSM-5 can be attributed to their stronger acidity compared to HY. | ||
| Fig. 1 Total FAME conversion using different zeolite catalysts: FFA was derived from (A) S. limacinum; (B) N. salina. | ||
In addition, an increase in the Si/Al framework molar ratio of zeolites increases their hydrophobicity.28 Hydrophilic zeolites absorb a considerable amount of water in esterification. Water content limits the maximum conversion that can be achieved. Thus, hydrophobic zeolites with high Si/Al, such as HZSM-5 and Hβ, are preferred for esterification.
Although the acidic strength of HZSM-5 is higher than Hβ, the catalytic activity of Hβ was superior in this case. The higher esterification conversion of Hβ vs. HZSM-5 might be due to larger pore size and pore configuration. The HZSM-5 zeolite had the smallest pore size of the zeolites tested in this study (Table 2). Furthermore, zeolite HZSM-5 consisted of two sets of channels: straight channels (elliptical cross section of about 5.4–5.6 Å) and sinusoidal channels (circular cross section of about 5.1–5.4 Å), providing strong shape-selective properties.29 Small pore size and zigzag shape affect mass transfer because the narrow pore opening of the HZSM-5 zeolite prevents the entry of FFA, particularly those of larger molecular weight and greater molecular volume. On the other hand, catalysts with larger pore size allow substrates (both FFA and methanol in this case) to enter the pore structure and react at a faster rate. Thus, the Hβ displayed the highest esterification yield, due to its high acidity and moderate pore size.
3.4 The selectivity of zeolites in esterification reaction
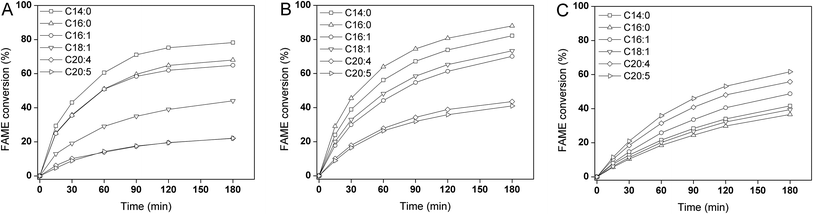
As shown in Fig. 2 and 3, zeolites selectively catalyzed different FFAs into FAME. The esterification selectivity followed the order of HZSM-5 > Hβ > HY. The zeolite catalysts with a smaller pore size tended to catalyze the esterification of SCFA, such as C14:0, C15:0, C16:0 and C18:1, while had a lower conversion to LCFA. The lower conversion of LCFA was caused by the longer chains, which resulted in higher steric hindrance. In addition, PUFA such as EPA and DHA have contorted structures, due to the presence of extensive unconjugated cis-double bonds. This provided these fatty acids with a circular shape, impeding the mass transfer of these FFAs in the porous catalyst. | ||
| Fig. 2 FAME conversion with zeolite catalysts: (A) HZSM-5; (B) Hβ; (C) HY. Feedstock FFA was derived from S. limacinum. | ||
 | ||
| Fig. 3 FAME conversion with zeolite catalysts: (A) HZSM-5; (B) Hβ; (C) HY. Feedstock FFA was derived from N. salina. | ||
As illustrated in Fig. 2a and 3a, the HZSM-5 showed an FFA conversion rate from S. limacinum in the following order of C14:0 > C15:0/C16:0 > C18:1 > C22:5/C22:6. Similar results were obtained for N. salina: C14:0 > C16:0/C16:1 > C18:1 > C20:4/C20:5. The esterification rates of the SCFA in both cases were consistently higher than that of LCFA due to steric hindrance.30
When zeolite Hβ was used for esterification (Fig. 2b and 3b), selectivity was generally higher for FFAs with shorter chains (C14:0, C15:0, C16:0 and C18:1) than those with longer chains (C20:5, C22:5 and C22:6). For FFA from S. limacinum, the conversion rate followed the order of C18:1 > C16:0 > C15:0 > C14:0 > C22:5 > C22:6. For N. salina, the conversion rate followed the order of C16:0 > C14:0 > C18:1 > C16:1 > C20:4 > C20:5. Although the Hβ could selectively catalyze SCFA into FAME, the selectivity was lower than HZSM-5. The HZSM-5 and Hβ exhibited a clear preference for converting the shorter chain FFAs to esters.
However, the HY catalyst exhibited opposite selectivity to the longer chain FFAs (Fig. 2c and 3c). For FFAs from S. limacinum, the conversion rate followed the order of C22:6 > C22:5 > C14:0 > C15:0 > C16:0 > C18:1. For N. salina, the conversion rate followed the order of C20:5 > C20:4 > C16:1 > C14:0 > C18:1 > C16:0. Since the zeolite HY had large pore size that might not limit access of complex FFA, the larger molecules such as C20:5, C22:5 and C22:6, might enter the porous structure and block the access of smaller FFAs to the active site on the inner surface. This reduced the esterification rate of smaller substrates. This selectivity is not desirable for biodiesel production, and therefore no further tests were conducted with this catalyst in this study.
Although all the zeolite catalysts exhibited selectivity to esterification of different FFAs, the selective patterns varied remarkably. The HZSM-5 and Hβ showed a clear preference to SCFAs, while HY favored the esterification of LCFAs. It might be the molecular weight and geometry configuration of FFA that affects the esterification catalyzed by zeolites. These results highlight the need for further studies on zeolite selectivity and the mechanisms of FFA esterification reactions.
3.5 Recovery rate of PUFA in unreacted FFA
As shown in Table 3, concentrations of DPA (C22:5), DHA (C22:6), EPA (C20:5) and ARA (C20:4) in unreacted FFA can increase two-fold, with recovery rates of 72%, 70%, 78% and 78% at 180 min, respectively. Although both of the concentrations of DPA and DHA can be further increased to 2.4 times with a longer reaction time (300 min), recovery rates decreased to 61%. This suggests that zeolite HZSM-5 can selectively convert FFA based on diffusion limitations; however, bulky FFA can still be esterified, perhaps on the external surface of the catalyst.31 In addition, as reaction time went on, SCFA and some impurities in the algal oil might block the microporous structure, reducing selectivity. To enhance the selectivity of zeolite HZSM-5, non-selective acid sites on the external surface can be inactivated with the deposition of carbonaceous and siliceous materials.32| S. limacinum | N. salina | |||||
|---|---|---|---|---|---|---|
| Enrich ratioa | FFA recoverya (%) | Enrich ratiob | FFA recoveryb (%) | Enrich ratioa | FFA recoverya (%) | |
a Reaction condition: 10 wt% HZSM-5; methanol to FFA ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; temperature 85 °C; reaction time a180 min, b300 min. 1; temperature 85 °C; reaction time a180 min, b300 min. |
||||||
| C14:0 | 0.37 | 11.9 | 0.27 | 7.0 | 0.58 | 21.8 |
| C15:0 | 0.43 | 13.8 | 0.29 | 7.4 | — | — |
| C16:0 | 0.44 | 14.2 | 0.34 | 8.8 | 0.86 | 32.0 |
| C16:1 | — | — | — | — | 0.94 | 35.1 |
| C18:1 | 0.54 | 17.4 | 0.44 | 11.2 | 1.50 | 55.9 |
| C20:4 | — | — | — | — | 2.09 | 78.0 |
| C20:5 | — | — | — | — | 2.09 | 77.8 |
| C22:5 | 2.23 | 72.1 | 2.4 | 61.5 | — | — |
| C22:6 | 2.19 | 71.0 | 2.4 | 61.5 | — | — |
It is very difficult to separate long-chain, high-value ω-3 fatty acids from other fatty acids on a large scale, due to their similar physical and chemical properties.10,33 The conventional methods used in the industry are not practical for processing a large amount of feedstocks, such as microalgae oils considered for biodiesel production. Selective esterification may be employed as a solution to this challenge. Previously, selective enzymatic hydrolysis and esterification were reported as efficient way to enrich PUFA from fish oil.34,35 However, lipases are expensive and the conversion efficiency was not high. Compared to lipase-catalysed selective reaction, zeolite is more efficient and cost-efficient. This novel process is not only suitable for large scale biofuel production, but also promising for nutraceutical production. The selective esterification process developed in this study allows integration of microalgal biodiesel production and high-value ω-3 PUFA fractionation. Following selective esterification, enriched PUFA in the unreacted FFA phase (Fig. 4 and 5) may be easily separated from FAME by existing industry infrastructures, such as ion-exchange chromatography column and molecular distillation to recover high-value ω-3 fatty acids as a co-product. The quality of biodiesel, especially oxidation stability, is likely enhanced due to the reduction of unstable PUFA. The co-production of high-value ω-3 fatty acids through the selective catalytic process may improve the economic viability of algal biodiesel production.
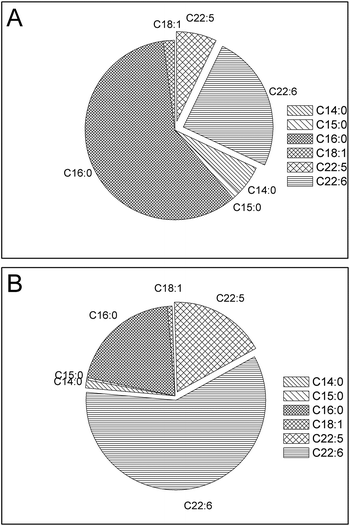 | ||
| Fig. 4 FFA composition in unreacted FFA stream before (A) and after (B) selective esterification of oil derived from S. limacinum. | ||
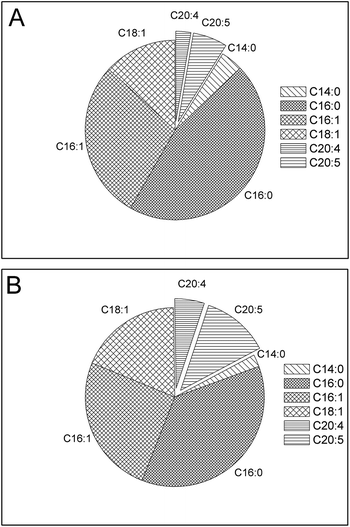 | ||
| Fig. 5 FFA composition in unreacted FFA stream before (A) and after (B) selective esterification of oil derived from N. salina. | ||
4. Conclusions
The integrated biodiesel production and PUFA enrichment process with selective zeolite as a catalyst (HZSM-5) preferentially converted low-value SCFA into FAME (biodiesel) but retained most of the PUFA in their original FFA form. It was demonstrated that the conversion of SCFA derived from S. limacinum and N. salina reached to 86% and 65%, respectively. In the meantime, the PUFA concentration in the unreacted FFA stream was increased more than 2 folds. The enrichment of PUFA in the FFA phase may lead to more efficient downstream separation. Moreover, the quality of biodiesel produced is likely enhanced due to the reduced PUFA content.Acknowledgements
This project was funded by the Bill & Melinda Gates Foundation through the Grand Challenges Exploration (Round 9).Notes and references
- A. C. Guedes, H. M. Amaro and F. X. Malcata, Biotechnol. Prog., 2011, 27, 597–613 CrossRef CAS PubMed.
- T. Dong, J. Wang, C. Miao, Y. Zheng and S. Chen, Bioresour. Technol., 2013, 136, 8–15 CrossRef CAS PubMed.
- P. Spolaore, C. Joannis-Cassan, E. Duran and A. Isambert, J. Biosci. Bioeng., 2006, 101, 87–96 CrossRef CAS PubMed.
- Z. Chi, Y. Liu, C. Frear and S. Chen, Appl. Microbiol. Biotechnol., 2009, 81, 1141–1148 CrossRef CAS PubMed.
- D. Pal, I. Khozin-Goldberg, Z. Cohen and S. Boussiba, Appl. Microbiol. Biotechnol., 2011, 90, 1429–1441 CrossRef CAS PubMed.
- T. Dong, L. Yu, D. Gao, X. Yu, C. Miao, Y. Zheng, J. Lian, T. Li and S. Chen, Appl. Microbiol. Biotechnol., 2015, 1–11, DOI:10.1007/s00253-015-6909-2.
- G. Wang and T. Wang, J. Am. Oil Chem. Soc., 2011, 89, 135–143 CrossRef.
- N. D. Riediger, R. a. Othman, M. Suh and M. H. Moghadasian, J. Am. Diet. Assoc., 2009, 109, 668–679 CrossRef CAS PubMed.
- J. Wang, X.-D. Wang, X.-Y. Zhao, X. Liu, T. Dong and F.-A. Wu, Bioresour. Technol., 2014, 184, 405–414, DOI:10.1016/j.biortech.2014.09.133.
- J. a. Kralovec, S. Zhang, W. Zhang and C. J. Barrow, Food Chem., 2012, 131, 639–644 CrossRef CAS PubMed.
- Z. S. Petrović, X. Wan, O. Bilić, A. Zlatanić, J. Hong, I. Javni, M. Ionescu, J. Milić and D. Degruson, J. Am. Oil Chem. Soc., 2013, 90, 1073–1078 CrossRef.
- K. D. Carlson and S. P. Chang, J. Am. Oil Chem. Soc., 1985, 62, 934–939 CrossRef CAS.
- N. U. Soriano and A. Narani, J. Am. Oil Chem. Soc., 2012, 89, 917–923 CrossRef CAS.
- A. R. Medina, E. M. Grima, A. G. Giménez and M. J. I. González, Biotechnol. Adv., 1998, 16, 517–580 CrossRef CAS.
- A. Corma, Chem. Rev., 1997, 97, 2373–2420 CrossRef CAS PubMed.
- T. Dong, D. Gao, C. Miao, X. Yu, C. Degan, M. Garcia-Pérez, B. Rasco, S. S. Sablani and S. Chen, Energy Convers. Manage., 2015, 105, 1389–1396, DOI:10.1016/j.enconman.2015.06.072.
- W. W. Christie, Isolation, Separation, Identification and Lipidomic Analysis, 3rd edn, 2003 Search PubMed.
- D. J. Pyle, R. a. Garcia and Z. Wen, J. Agric. Food Chem., 2008, 56, 3933–3939 CrossRef CAS PubMed.
- P. M. Kris-Etherton, W. S. Harris, L. J. Appel and N. Comm, Circulation, 2002, 106, 2747–2757 CrossRef PubMed.
- M. J. Ramos, C. M. Fernández, A. Casas, L. Rodríguez and Á. Pérez, Bioresour. Technol., 2009, 100, 261–268 CrossRef CAS PubMed.
- M. Mittelbach, Bioresour. Technol., 1996, 56, 7–11 CrossRef CAS.
- V. J. Frillette, W. O. Haag and R. M. Lago, J. Catal., 1981, 67, 218–222 CrossRef CAS.
- IZA, http://www.iza-online.org/.
- M. M. J. Treacy and M. D. Foster, Microporous Mesoporous Mater., 2009, 118, 106–114 CrossRef CAS PubMed.
- S. I. Zones and T. V. Harris, Microporous Mesoporous Mater., 2000, 35–36, 31–46 CrossRef CAS.
- Y. F. Chu, US Pat., 4,927,525, 1990.
- Y. Miyamoto, N. Katada and M. Niwa, Microporous Mesoporous Mater., 2000, 40, 271–281 CrossRef CAS.
- M. Sasidharan and R. Kumar, J. Mol. Catal. A Chem., 2004, 210, 93–98 CrossRef CAS PubMed.
- Y. Fan, X. Bao, X. Lin, G. Shi and H. Liu, J. Phys. Chem. B, 2006, 110, 15411–15416 CrossRef CAS PubMed.
- M. D. S. Machado, J. Pérez-Pariente, E. Sastre, D. Cardoso and A. M. de Guereñu, Appl. Catal., A, 2000, 203, 321–328 CrossRef CAS.
- D. E. López, J. G. Goodwin, D. a. Bruce and E. Lotero, Appl. Catal., A, 2005, 295, 97–105 CrossRef PubMed.
- F. Bauer, W. H. Chen, E. Bilz, a. Freyer, V. Sauerland and S. B. Liu, J. Catal., 2007, 251, 258–270 CrossRef CAS PubMed.
- M. Alkio, C. Gonzalez, M. Jäntti and O. Aaltonen, J. Am. Oil Chem. Soc., 2000, 77, 315–321 CrossRef CAS.
- Y. Shimada, A. Sugihara and Y. Tominaga, J. Biosci. Bioeng., 2001, 91, 529–538 CrossRef CAS.
- Y. Shimada, Y. Watanabe, A. Kawashima, K. Akimoto, S. Fujikawa, Y. Tominaga and A. Sugihara, J. Am. Oil Chem. Soc., 2003, 80, 37–42 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |
