Tailoring the surface properties of cerium oxide nanoabrasives through morphology control for glass CMP
Abstract
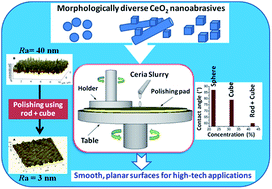
Nanosized cerium oxide (CeO2) particles possessing different morphologies like nanorods, nanocubes and nanospheres have been successfully synthesized by a simple one step, surfactant free, precipitation technique and by hydrothermal methods. The diverse morphology motifs were further utilized for the planarization of silicate glass with an initial surface roughness of ∼40 nm and we observed a strong morphology dependence of the abrasive in glass polishing. Polishing efficiency of the nanoabrasives in terms of the mass removal and surface roughness was investigated using a table top lapping machine. Surface roughness analysis by atomic force microscopy reveals that the ceria nanostructure with a mixed morphology of rods and cubes could produce a surface finish of ∼3 Å. The surface properties of the abrasive were found to play a key role in polishing as evidenced by X-ray photoelectron spectroscopy and Raman spectral analysis. The powder contact angle/hydrophilicity of the nanopowders followed the same trend as that of the dipolar (200) plane and [Ce3+]. This work has shown promise in polishing efficiency with nano CeO2 slurry to achieve nanolevel planarity on glass substrates, which is desirable for the global planarization of complex device topography.

 Please wait while we load your content...
Please wait while we load your content...