Insights into the mechanism and kinetics of the gas-phase atmospheric reaction of 9-chloroanthracene with NO3 radical in the presence of NOx†
Juan Dang,
Xiangli Shi,
Qingzhu Zhang*,
Jingtian Hu and
Wenxing Wang
Environment Research Institute, Shandong University, Jinan 250100, P. R. China. E-mail: zqz@sdu.edu.cn; Fax: +86-531-8836 1990
First published on 30th September 2015
Abstract
9-Chloroanthracene (9-ClAnt), an important member of the chlorinated polycyclic aromatic hydrocarbons (ClPAHs), has been demonstrated to show strong direct mutagenic effects. To elucidate the chemical transformations, degradation products and the capacity to undergo long-range transport of 9-ClAnt in the atmosphere, we conducted a theoretical investigation into the oxidation mechanism and kinetics of the NO3-initiated atmospheric transformation of 9-ClAnt by using a quantum chemistry method. The rate constants for the crucial elementary reactions were also estimated. The main oxidation products for the gas-phase reactions of 9-ClAnt with NO3 radicals include 9-chloroanthracen-yl nitrates, 9-chloroanthracenesdiones, epoxides, dialdehydes, 9-chloroanthracene-1,4-dione, anthracene-9,10-dione, 9-chloroanthracen-1-one, 10-chloroanthracen-1-one, 10-chloroanthracen-9-ol and 10-chloro-1-nitroanthracene etc. The overall rate constant of the NO3 addition reactions is 9.11 × 10−13 cm3 molecule−1 s−1 at 298 K and 1 atm. The atmospheric lifetime of 9-ClAnt determined by using NO3 radicals is about 0.61 h. This comprehensive investigation is the first report for the NO3-initiated oxidation of ClPAHs in the atmosphere. It should be conducive to clarifying their atmospheric fates and developing a full understanding of their toxic potential.
1. Introduction
Chlorinated polycyclic aromatic hydrocarbons (ClPAHs) have attracted considerable interest because of their ubiquitous presence in environmental matrices and potential dioxin-like toxicity.1–4 ClPAHs can elicit toxic effects via hindering the function of the aryl hydrocarbon receptor (AhR).5 Automobile exhaust and municipal incineration have been perceived as major emission sources of ClPAHs.6 Compared with their parent polycyclic aromatic hydrocarbons (PAHs), ClPAHs have lower vapor pressures, higher octanol–water partition coefficient values, and even possess stronger mutagenicity and toxic effects.4,7 ClPAHs have been constantly detected in the atmosphere and the concentration in urban air was up to 143 pg m−3 in Japan.8 Due to the scarcity of purified individual ClPAHs standards, the environmental behavior and fate of these compounds in the atmosphere have not been fully clarified.9-chloroanthracene (9-ClAnt), a chloro-substituted anthracene derivative, has been demonstrated to show strong direct mutagenic effects.9,10 According to the report, 9-ClAnt has been frequently detected in the atmospheric monitoring. In December 2009, air samples collected from the urban area of Shizuoka in Japan showed that the maximum concentration of 9-ClAnt was 12.10 pg m−3.11 The measurement in a Japanese urban city from December 2004 to December 2005 indicated that ambient air concentrations of 9-ClAnt ranged from <0.02 to 71 pg m−3.12 Given its toxicity and prevalent presence in air, little is known about the atmospheric fate of 9-ClAnt.
Under typical ambient conditions, 9-ClAnt exists mainly in the gas phase and can be removed from the atmosphere by gas-phase reactions with OH radicals, NO3 radicals and O3.7,13 The reaction with OH radicals is an important loss process during daylight hours, and reaction with NO3 radicals is dominant chemical transformations during the evening and nighttime.13 Compared to the reaction with OH, the reaction of PAHs with NO3 appears to be less significant. However, considering the higher yields of nitro-PAH in the nighttime, it suggests that the reaction with NO3 may also play an important role.14 To date, no information on the NO3-initiated atmospheric oxidation of ClPAHs (9-ClAnt included) is available in previous studies. Besides, a complete analysis of the atmospheric reactions is limited in the laboratory studies, largely due to the lack of efficient detection schemes for intermediate radical species. Hence we carried out a comprehensive theoretical investigation on the NO3-initiated atmospheric oxidation of 9-ClAnt in the presence of O2/NOx, using the quantum chemistry calculation and the energy grained master equation.15–17 The reaction mechanisms of the NO3-initiated atmospheric oxidation of 9-ClAnt and the rate constants for the key elementary reactions were obtained.
2. Computational method
The electronic structure calculations were performed with the Gaussian 09 software program suite.18 The hybrid density functional theory (DFT), BB1K, can give excellent transition state geometries and barrier heights, based on Becke's 1988 gradient corrected exchange functional (Becke88 or B) and Becke's 1995 kinetic-energy-dependent dynamical correlation functional (Becke95 or B95).19 Truhlar et al. indicated that the hybrid DFT results are much less sensitive to the basis set compared to the ab initio ones, and they also emphasized the importance of diffuse functions.20,21 According to the assessment of different methods by Zheng et al., the mean signed errors and mean unsigned errors for the DBH24/08 database as well as the errors for its components: heavy-atom transfer (HATBH6), nucleophilic substitution (NSBH6), unimolecular and association (UABH6), and hydrogen-transfer (HTBH6) reactions indicated that BB1K/6-31+G(d,p) have a comparatively high accuracy.22 Therefore geometries of the reactants, intermediates, transition states, and products were optimized at the BB1K/6-31+G(d,p) level. The transition states were identified with one imaginary frequency. The harmonic frequencies calculations were also performed at the same level in order to determine the character of the stationary points, the zero-point energy (ZPE), and the thermal contribution to the free energy of activation. Besides, the intrinsic reaction coordinate (IRC) analysis was executed to confirm that each transition state connects to the right minima along the reaction path. For a more accurate evaluation of the energetic parameters, a more flexible basis set 6-311+G(3df,2p), was employed to determine the single point energies of various species. The profile of the potential energy surface (PES) was constructed at the BB1K/6-311+G(3df,2p)//BB1K/6-31G+G(d,p) level. All the total electronic energies were corrected by the zero-point energy.All calculations for the rate constants have been performed by means of the MESMER program,17 MESMER uses matrix techniques to formulate and solve the energy grained master equation (EGME) for unimolecular systems. The exponential down model is used for describing collisional energy transfer probabilities. The expression is:23
 | (1) |
The population distribution of the reactants and intermediates on the potential energy surface (PES) was calculated by solving coupled differential equations that depict collisional energy transfer and interconversion between the species.24–26 The coupled set of differential equations may be expressed as:
 | (2) |
For unimolecular reactions with a well-defined transition state, the most common way of obtaining energy resolved microcanonical rate constants for a particular energy grain, k(E), were determined by using Rice–Ramsperger–Kassel–Marcus (RRKM) theory. The RRKM expression is given by:24
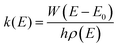 | (3) |
If no transition state is specified, an inverse Laplace transform (ILT) offers a mathematical formalism for deriving k(E)s from an Arrhenius fit to a set of k(E)s. The basis of the ILT is the standard (high-pressure limit) Boltzmann average which may be expressed as:24
 | (4) |
For a barrierless association reaction, considering the reverse unimolecular dissociation of the collision adduct, the forward (association) and reverse (dissociation) rate constants are related by the equilibrium constant. So the rate constant of the forward (association) reaction can be derived from the equilibrium constant and the rate constant of the reverse (dissociation) reaction.24
3. Results and discussion
3.1 Reaction with NO3 radicals
As shown in Fig. 1 and S1 (ESI†), the carbon atoms in the molecular of 9-ClAnt are numbered. Addition of NO3 to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and H abstraction from the C–H bonds by NO3 radicals are possible pathways for the reaction of 9-ClAnt with NO3 radicals. At the BB1K/6-311+G(3df,2p) level, the potential barriers of H abstractions are 39.33–57.78 kJ mol−1 and all reaction channels are endothermic by 31.00–36.19 kJ mol−1. The geometrical structures of the transition states for the H abstractions by NO3 radicals are shown in Fig. S4.† For the NO3 additions, the reactions are barrierless and the reaction heats are from −62.17 to −113.59 kJ mol−1. Clearly, the NO3 additions are the energetically more favorable reaction pathways for the reaction of 9-ClAnt with NO3 radicals, which is consistent with the previous investigations of the NO3-initiated atmospheric oxidations of unsubstituted PAHs.27,28 The NO3 addition reactions lead to the formation of NO3-9-ClAnt adducts.
C bonds and H abstraction from the C–H bonds by NO3 radicals are possible pathways for the reaction of 9-ClAnt with NO3 radicals. At the BB1K/6-311+G(3df,2p) level, the potential barriers of H abstractions are 39.33–57.78 kJ mol−1 and all reaction channels are endothermic by 31.00–36.19 kJ mol−1. The geometrical structures of the transition states for the H abstractions by NO3 radicals are shown in Fig. S4.† For the NO3 additions, the reactions are barrierless and the reaction heats are from −62.17 to −113.59 kJ mol−1. Clearly, the NO3 additions are the energetically more favorable reaction pathways for the reaction of 9-ClAnt with NO3 radicals, which is consistent with the previous investigations of the NO3-initiated atmospheric oxidations of unsubstituted PAHs.27,28 The NO3 addition reactions lead to the formation of NO3-9-ClAnt adducts.
3.2 Secondary reactions
The NO3-9-ClAnt adducts are resonantly stabilized radicals. According to the nodal pattern of the Hückel theory, the single occupied molecular orbital (SOMO) for the NO3-9-ClAnt adduct only has combinations on the ortho- or para-positions of –NO3 moiety. As depicted in Fig. 3, for IM1, O2 can attack the C atoms from the ortho- and para-positions of nitryl to produce two O2–NO3-9-ClAnt adducts (IM15 and IM19). The sequential reaction of IM15 contains four processes: NO barrierless association, elimination of NO2, cleavage of the C–C bond and loss of NO2. There into, the formation of IM17 is the rate-determining step with a high potential barrier of 199.11 kJ mol−1. A dialdehyde (P8) is formed from these reactions. Analogous products dialdehydes have been detected in the simulation chamber experiments of gas-phase atmospheric oxidation of acenaphthylene with NO3 radicals.29 The subsequent reaction of IM19 starts with NO addition, followed by elimination of NO2, H abstraction by O2, H abstraction by OH radical and loss of NO2, leading to the formation of 9-chloroanthracene-1,4-dione (P11). The calculated profile of the potential energy surface shows that the step of the O–ONO bond cleavage is difficult to occur because of the high potential barrier of 191.58 kJ mol−1. IM2 also can react with O2, proceeding with NO addition, cleavage of the O–ONO bond, C–C bond rupture and elimination of NO2, and it ultimately results in the formation of a dialdehyde (P8). The subsequent reaction from IM6 includes six elementary reactions: O2 addition, NO addition, O–ONO bond fission, elimination of Cl, H abstraction by OH radical and elimination of NO2, which lead to the generation of anthracene-9,10-dione (P12).
As shown in Fig. 5, IM1 can undergo cyclization to produce the intermediate IM48. The subsequent reactions from IM48 are two different N–O bonds fissions and result in the formation of IM49 and IM52. Then, 9-chloroanthracen-1-one (P13) and 10-chloroanthracen-1-one (P14) can be formed from the subsequent reactions of IM52 and IM49 through NO2 elimination, respectively. Another reaction channel of IM49 includes H abstraction by O2, H abstraction by OH radical and loss of NO with a rearrangement, which lead to the formation of 9-chloroanthracene-1,4-dione (P11). Calculations show that the formation of the intermediate IM48 is the rate-determining step due to its high potential barrier. Similarly, IM6 also can proceed with isomerization to form IM53, followed by cleavage of the N–O bond and rearrangement. The intermediate IM55 is generated from these processes. Subsequently, 10-chloroanthracen-9-ol (P15) can be formed by IM55 through loss of NO2. Another reaction pathway of IM55 contains two elementary reactions: elimination of ClNO and H abstraction by O2, which yield anthracene-9,10-dione (P12). Similar products, 1,4-naphthoquinone, have been detected in the experimental studies of the reaction of naphthalene with NO3 radicals.27 Calculations show that both of the two pathways are the energetically favorable processes for the further reactions of IM55.
3.3 Rate constant calculations
The rate constants for the critical elementary reactions involved in the NO3-initiated atmospheric reactions of 9-ClAnt were estimated by using the MESMER program.17 The MESMER program has been successfully applied to calculate the rate constants of many reactions.24,26 For the elementary reactions with well-defined transition states, the SimpleRRKM method was used to obtain the rate constants at 298 K and 1 atm, while the MesmerILT method was applied to calculate the rate coefficients for the barrierless reactions at 298 K and 1 atm. As shown in the Table 1, the individual rate constants for the NO3 addition to the C1–H, C2–H, C3–H, C4–H, C9–H and C10–H bonds of 9-ClAnt are denoted as k1, k2, k3, k4, k5, k6, respectively. Considering the structural symmetry of 9-ClAnt, the overall rate constant of the NO3 addition reactions is denoted as k, k = (k1 + k2 + k3 + k4) × 2 + k5 + k6. The overall rate constant k is calculated to be 9.11 × 10−13 cm3 molecule−1 s−1 at 298 K and 1 atm. The rate constants of the crucial secondary reactions of NO3-9-ClAnt adducts were also calculated, which can be helpful to the establishment of the kinetic models describing the atmospheric fate of 9-ClAnt. From the Table 1, it can be clearly observed that the formation of 10-chloro-1-nitroanthracene (P18) from IM59 is much easier than the generation of 9-chloro-2-nitroanthracene (P16) from IM57.| Reactions | Rate constants |
|---|---|
| 9-ClAnt + NO3 → NO3-9-ClAnt | (k) 9.11 × 10−13 |
| 9-ClAnt + NO3 → IM1 | (k1) 9.80 × 10−14 |
| 9-ClAnt + NO3 → IM2 | (k2) 8.69 × 10−14 |
| 9-ClAnt + NO3 → IM3 | (k3) 7.45 × 10−14 |
| 9-ClAnt + NO3 → IM4 | (k4) 9.81 × 10−14 |
| 9-ClAnt + NO3 → IM5 | (k5) 9.93 × 10−14 |
| 9-ClAnt + NO3 → IM6 | (k6) 9.69 × 10−14 |
| IM1 → IM15 | 3.48 × 10−20 |
| IM15 → IM16 | 5.21 × 10−13 |
| IM16 → IM17 | 6.92 × 10−23 |
| IM17 → IM18 | 1.08 × 10−2 |
| IM18 → P8 | 6.21 × 108 |
| IM1 → IM19 | 6.93 × 10−21 |
| IM19 → IM20 | 5.28 × 10−13 |
| IM20 → IM21 | 5.68 × 10−23 |
| IM23 → P11 | 2.10 × 108 |
| IM2 → IM24 | 1.48 × 10−18 |
| IM24 → IM25 | 5.23 × 10−13 |
| IM25 → IM26 | 1.87 × 10−22 |
| IM26 → IM27 | 2.64 × 10−1 |
| IM27 → P8 | 2.31 × 108 |
| IM1 → IM33 | 3.75 × 10−9 |
| IM33 → IM34 | 1.51 × 106 |
| IM34 → IM35 | 1.92 × 104 |
| IM35 → P8 | 7.99 × 108 |
| IM33 → IM36 | 1.43 × 106 |
| IM36 → IM37 | 5.93 × 103 |
| IM37 → P8 | 5.77 × 108 |
| IM57 → P1 | 4.29 × 10−21 |
| IM57 → P16 | 1.32 × 10−21 |
| IM59 → P3 | 1.10 × 10−18 |
| IM59 → P18 | 2.08 × 10−13 |
The atmospheric lifetime of 9-ClAnt determined by NO3 can be derived from the overall rate constant (k) of the NO3 addition reactions and the NO3 concentration ([NO3]). An average 12 h nighttime value for NO3 concentration in the low troposphere is approximately 5.0 × 108 molecule cm−3,29 from the expressions: 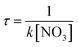 , the atmospheric lifetime (τ) of 9-ClAnt determined by NO3 is calculated to be 0.61 h, which indicate that 9-ClAnt can be removed from the atmosphere quickly and have less potential to undergo long-range transport to remote areas.
, the atmospheric lifetime (τ) of 9-ClAnt determined by NO3 is calculated to be 0.61 h, which indicate that 9-ClAnt can be removed from the atmosphere quickly and have less potential to undergo long-range transport to remote areas.
4. Conclusions and environmental perspectives
In this work, we conducted an extensive theoretical study on the reaction mechanism and kinetics of the NO3 radical-initiated atmospheric oxidation of 9-ClAnt. The rate constants for the crucial elementary reactions were evaluated by using the MESMER program. The following specific conclusions can be summarized from this study:(1) Compared with H abstractions by NO3 radicals, the NO3 additions are the energetically more favorable reaction pathways for the reaction of 9-ClAnt with NO3 radicals. The NO3 addition reactions are barrierless and the reaction heats range from −62.17 to −113.59 kJ mol−1.
(2) The NO3-initiated atmospheric oxidation of 9-ClAnt generates a class of anthracene derivatives containing 9-chloroanthracen-yl nitrates, 9-chloroanthracenesdiones, epoxides, dialdehydes, 9-chloroanthracene-1,4-dione, anthracene-9,10-dione, 9-chloroanthracen-1-one, 10-chloroanthracen-1-one, 10-chloroanthracen-9-ol and 10-chloro-1-nitroanthracene etc.
(3) The overall rate constant for the NO3 additions to 9-ClAnt is 9.11 × 10−13 cm3 molecule−1 s−1 at 298 K and 1 atm. The atmospheric lifetime of 9-ClAnt determined by NO3 radicals is about 0.61 h.
Although the concentrations of ClPAHs in the atmosphere have been detected to be a lower level than those of PAHs, some may be higher than those of polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and polychlorinated biphenyls (PCBs). To date, the investigations on the chemical transformations of ClPAHs in the atmosphere including homogeneous reactions and heterogeneous reactions have been more limited than those of other toxic persistent pollutants, such as PCDD/Fs and PAHs. However, the atmospheric reactions of ClPAHs appear to differ from those of PCDD/Fs and PAHs, and we should therefore be focused on the elucidation for the reaction mechanisms, degradation products and the capacity to undergo long-range transport of ClPAHs in the atmosphere. Thus, we can develop a comprehensive and accurate understanding of their toxic potential.
Acknowledgements
The work was financially supported by NSFC (National Natural Science Foundation of China, project No. 21337001, 21377073).References
- K. Kakimoto, H. Nagayoshi, Y. Konishi, K. Kajimura, T. Ohura, K. Hayakawa and A. Toriba, Chemosphere, 2014, 111, 40–46 CrossRef CAS PubMed.
- A. Kitazawa, T. Amagai and T. Ohura, Environ. Sci. Technol., 2006, 40, 4592–4598 CrossRef CAS.
- K. Sankoda, K. Nomiyama, T. Yonehara, T. Kuribayashi and R. Shinohara, Chemosphere, 2012, 88, 542–547 CrossRef CAS PubMed.
- K. Sankoda, T. Kuribayashi, K. Nomiyama and R. Shinohara, Environ. Sci. Technol., 2013, 47, 7037–7044 CAS.
- T. Ohura, Sci. World J., 2007, 7, 372–380 CrossRef PubMed.
- J. Ma, Z. Y. Chen, M. H. Wu, J. L. Feng, Y. Horii, T. Ohura and K. Kannan, Environ. Sci. Technol., 2013, 47, 7615–7623 CrossRef CAS PubMed.
- J. L. Sun, H. Zeng and H. G. Ni, Chemosphere, 2013, 90, 1751–1759 CrossRef CAS PubMed.
- Y. Horii, G. Ok, T. Ohura and K. Kannan, Environ. Sci. Technol., 2008, 42, 1904–1909 CrossRef CAS.
- D. L. Wang, X. B. Xu, S. G. Chu and D. R. Zhang, Chemosphere, 2003, 53, 495–503 CrossRef CAS.
- U. L. Nilsson and C. E. Oestman, Environ. Sci. Technol., 1993, 27, 1826–1831 CrossRef CAS.
- T. Ohura, Y. Horii, M. Kojima and Y. Kamiya, Atmos. Environ., 2013, 81, 84–91 CrossRef CAS PubMed.
- T. Ohura, S. Fujima, T. Amagai and M. Shinomiya, Environ. Sci. Technol., 2008, 42, 3296–3302 CrossRef CAS.
- R. Atkinson and J. Arey, Chem. Rev., 2003, 103(12), 4605–4638 CrossRef CAS PubMed.
- I. J. Keyte, R. M. Harrison and G. Lammel, Chem. Soc. Rev., 2013, 42, 9333–9391 RSC.
- G. P. Robert, Annu. Rev. Phys. Chem., 1983, 34, 631–656 CrossRef.
- P. J. Robinson and K. A. Holbrook, Unimolecular Reactions, Wiley, New York, 1972 Search PubMed.
- S. H. Robertson, D. R. Glowacki, C.-H. Liang, C. Morley, M. J. Pilling, MESMER (Master Equation Solver for Multi-Energy Well Reactions), 2008, an object oriented C++ program for carrying out ME calculations and eigenvalue-eigenvector analysis on arbitrary multiple well systems. http://sourceforge.net/projects/mesmer.
- M. Frisch, G. Trucks, H. B. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, B. Mennucci and G. Petersson, Gaussian 09, revision A. 02, Gaussian. Inc., Wallingford, CT, 2009, vol. 270, p. 271 Search PubMed.
- Y. Zhao, B. J. Lynch and D. G. Truhlar, J. Phys. Chem. A, 2004, 108(14), 2715–2719 CrossRef CAS.
- B. J. Lynch and D. G. Truhlar, J. Phys. Chem. A, 2001, 105(13), 2936–2941 CrossRef CAS.
- B. J. Lynch, Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2003, 107(9), 1384–1388 CrossRef CAS.
- J. J. Zheng, Y. Zhao and D. G. Truhlar, J. Chem. Theory Comput., 2009, 5, 808–821 CrossRef CAS.
- M. J. Pilling and S. H. Robertson, Master equation models for chemical reactions of importance in combustion, Annu. Rev. Phys. Chem., 2003, 54, 245–275 CrossRef CAS PubMed.
- D. R. Glowacki, C. H. Liang, C. Morley, M. J. Pilling and S. H. Robertson, J. Phys. Chem. A, 2012, 116, 9545–9560 CrossRef CAS PubMed.
- S. H. Robertson, M. J. Pilling, L. C. Jitariu and I. H. Hillier, Phys. Chem. Chem. Phys., 2007, 9(31), 4085–4097 RSC.
- J. Zhou, J. W. Chen, C. H. Liang, Q. Xie, Y. N. Wang, S. Y. Zhang, X. L. Qiao and X. H. Li, Environ. Sci. Technol., 2011, 45, 4839–4845 CrossRef CAS PubMed.
- X. H. Qu, Q. Z. Zhang and W. X. Wang, Chem. Phys. Lett., 2006, 432, 40–49 CrossRef CAS PubMed.
- R. Atkinson, J. Phys. Chem. Ref. Data, 1991, 20, 459–507 CrossRef CAS PubMed.
- S. M. Zhou and J. C. Wenger, Atmos. Environ., 2013, 75, 103–112 CrossRef CAS PubMed.
- J. Sasaki, S. M. Aschmann, E. S. C. Kwok, R. Atkinson and J. Arey, Environ. Sci. Technol., 1997, 31, 3173–3179 CrossRef CAS.
- J. Dang, X. L. Shi, Q. Z. Zhang, J. T. Hu and W. X. Wang, Sci. Total Environ., 2015, 505, 787–794 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The H abstraction reaction scheme of 9-ClAnt. The reaction schemes of IM1, IM2, IM3 and IM4. The isomerization unimolecular decomposition reaction scheme of the NO3-9-ClAnt adducts. Configuration for the transition states of H abstractions from 9-ClAnt optimized at the BB1K/6-31+G(d,p) level of theory. Distances are in angstrom. See DOI: 10.1039/c5ra11918a |
| This journal is © The Royal Society of Chemistry 2015 |






