Fabrication of superhydrophobic surfaces by smoke deposition and application in oil–water separation
Wenlian
Qiu
a,
Du
Xu
a,
Bin
Liu
a,
Lie
Shen
*a and
Qipeng
Guo
b
aMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: shenlie@zju.edu.cn
bPolymers Research Group, Institute for Frontier Materials, Deakin University, Locked Bag 20000, Geelong, Victoria 3220, Australia. E-mail: qipeng.guo@deakin.edu.au
First published on 12th August 2015
Abstract
Utilizing the smoke emitted by discarded silicone combustion, a simple method of smoke deposition is presented for fabricating a superhydrophobic surface with outstanding water repellence, which exhibited a water contact angle of 164 ± 0.8° and a sliding angle of lower than 1°. In addition, the as-prepared surface possesses favourable heat, water impact and water immersion stabilities. Oil leakages seriously endanger both the environment and the social economy. By this simple smoke deposition method, a selective-wettability copper mesh has been fabricated to separate oil–water mixtures. The smoke-deposited mesh achieved a high separation efficiency of over 93% for various oils, and showed excellent reusability, maintaining a high separation efficiency over 10 cycles. The water repellence of the used mesh can be refreshed by recoating with silicone and smoke deposition. This work provides a new strategy to utilize discarded silicone to fabricate superhydrophobic surfaces and oil–water separation meshes.
Introduction
Wettability is an important feature of solid surfaces and is determined by the chemical composition and the geometrical structure of the surface.1–3 A surface exhibiting a high water contact angle (WCA) of greater than 150° and a low water drop sliding angle of less than 10° is defined as a superhydrophobic surface. Low surface energy and rough structure induce a high hydrophobicity.4 The creation of superhydrophobic surfaces has drawn great interest in both scientific research and practical applications5,6 for their extensive uses in self-cleaning,7 solar cell panels,8 responsive smart materials9 and oil–water separation.10 In the past few decades, numerous methods and techniques for fabricating artificial superhydrophobic surfaces have been developed,11 mostly focusing on structuring micro- and nano-scale combined surfaces since low surface energy materials are limited.Siloxane rubber possesses hydrophobicity combined with a wide range of other excellent properties such as long-term stability, low toxicity and transparency. It is widely used in lots of fields including electric insulators, fluidics, anti-fouling coatings, biomedical applications, encapsulation and sealing rings.12,13 The most-used siloxane rubber is based on polydimethylsiloxane (PDMS), which is attractive for the fabrication of superhydrophobic materials due to its intrinsic hydrophobicity. Cortese et al. created microstructured pillars on PDMS surfaces via replication from a self-prepared mold and subsequent treatment of the samples in CF4 plasmas to generate submicrometer features on the top of the pillars, endowing the PDMS surfaces with superhydrophobicity.14 Crick et al. combined PDMS and nanoparticles with an aerosol-assisted chemical vapour deposition (CVD) approach to obtain a rough surface structure and the property of superhydrophobicity.15 Yong et al. employed a point-by-point femtosecond laser scanning process to produce microwell arrays on PDMS films. The as-prepared surfaces showed not only superhydrophobicity, but also water-controllable adhesion that ranged from ultrahigh to ultralow, by adjusting the extent of overlap of the adjacent microwells, on which the sliding angle can be controlled from 180° to 3°.16
Owing to the natural hardness, good gas barrier properties and high thermal stability properties of silicon oxide, numerous investigations on the growth of a silicon oxide surface layer on a polysiloxane film have arisen to explore their application in gas separation membranes, protective coatings for medical devices and microelectronics.17 Various fabrication techniques and methods have been developed, such as oxygen plasma, CVD, laser irradiation and UV-ozone methods.17–19 However, research on fabricating superhydrophobic materials utilizing siloxane to obtain the silicon oxide layer has seldom been reported, not to mention the oxidation method of directly burning. We have previously studied the creation of superhydrophobic coatings utilizing silicone combustion product.20 Herein, we report the fabrication of superhydrophobic surfaces utilizing another substance produced from the combustion process, which will realize making full use of discarded silicone.
Oil leakage incidents have a significant influence on both the environment and the social economy. Regardless of the immediate danger to marine species and ecosystems, there exists a long-lasting threat to ecological environments and economic development.21 Researchers have been exploring new oil–water separation and recovery methods with convenience, high efficiency, low cost, environmental friendliness and easy fabrication. Superhydrophobic and superoleophilic materials have received extensive attention in the application of oil–water separation and can be mainly classified into two categories, i.e. oil absorbent materials and oil–water separating mesh/film materials. The substrates of oil absorbent materials are mostly porous sponges, foams and aerogels.22–24 They can selectively absorb oil from oil–water mixtures and regain the oil through the simple process of compression. Oil–water separation mesh/film materials purify water and collect oil by the approach of filtration. Jiang et al. first reported the fabrication of a superhydrophobic and superoleophilic stainless steel mesh by a spray method, constructing a material with a nano-scale crater-like surface and low surface energy.25 The work has inspired a great interest in the field of oil–water separation materials fabricated by modifying the surfaces of meshes/films. Wang et al. fabricated a superhydrophobic and superoleophilic copper mesh through a thermal oxidation process, which was then designed as a box-like oil collector, realizing rapid oil recovery with a high separation efficiency.26 Lin et al. etched copper meshes with nitric acid followed by modification with 1-hexadecanethiol, and the meshes could quickly separate a diesel oil and water mixture.27 Zhou et al. incorporated polyaniline and fluorinated alkyl silane into cotton fabric via vapor-phase deposition, endowing the fabric with superhydrophobicity and superoleophilicity; it was applied in oil–water separation with an efficiency of 97.8% and a reusability of more than 30 times.28
However, to some extent, the existing methods have defects such as complex processes, expensive materials such as fluorinated materials, hazardous solvents and long processing times. In this study, an easy smoke deposition approach is described that can be applied in different substrates. Based on the above-mentioned oil–water separation studies, we applied the method to prepare superhydrophobic and superoleophilic surfaces on copper meshes, which can separate various oils and organic solvents with outstanding reusability.
Experimental section
Materials
Silicone bowl lids, which were commodities commercially available and recycled from daily items, were cut into strips with a cross-sectional size of about 3 mm × 5 mm. Sylgard 184 (polydimethylsiloxane) with a curing agent (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio) was purchased from Dow Corning. Commercial glass slides and copper meshes were initially cleaned by detergent, water and ethanol. Silica (SiO2, 20 nm) was obtained from Aladdin.
1 weight ratio) was purchased from Dow Corning. Commercial glass slides and copper meshes were initially cleaned by detergent, water and ethanol. Silica (SiO2, 20 nm) was obtained from Aladdin.
Preparation of superhydrophobic coatings on glass substrates
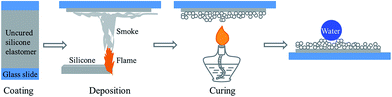
As shown in Scheme 1, the superhydrophobic silicone coatings on glass substrates were prepared by the following steps. First, Sylgard 184 was coated on the glass substrates, forming uncured silicone coatings with thicknesses of 4–8 µm. Subsequently, the uncured silicone coatings were exposed to the white smoke emitted from combustion of the silicone strips, at a height of about 40 mm. During the exposure, the particles in the white smoke were deposited on the uncured silicone coatings, leading the silicone coatings to change from transparent to white. The silicone strips were fixed, enabling the smoke to be steady. Moving the glass slides back and forth, the deposition was maintained until the silicone coatings were uniformly deposited with the white particles. Afterwards, the deposited silicone coatings were exposed to an alcohol lamp flame, at a height of 10 mm above for 15 s, to cure the silicone coatings thoroughly (or the coatings were cured at 100 °C for 20 min). The obtained coatings were then impacted with a water column for 10 s to remove the unbound particles. The samples without deposition were directly exposed to an alcohol lamp flame, at a height of 10 mm above for 15 s, to cure the silicone coatings.Superhydrophobic copper meshes and oil/water separation
Both sides of copper mesh sheets (60 × 60 mm) were coated with Sylgard 184. In order to ensure that mesh pores were unblocked, a brush was used to coat to obtain a thin silicone coating, and subsequently air was blown into the mesh pores with an air pump. After that, both sides of the copper mesh sheets coated with uncured silicone were exposed to the white smoke coming from the silicone strips combustion at a height of 40 mm, during which the coatings turned white, from their original coppery-yellow color. Moving the copper mesh sheets around, deposition was continued until the silicone coatings were uniformly deposited with the white particles. The resultant mesh sheets were then cured by moving them around at the position of 10 mm above an alcohol lamp flame for 15 s (or the coatings were cured at 100 °C for 20 min). Finally, the unbound particles on the copper mesh sheets were removed by blowing air with an air pump, and the white faded out. The oils used in this study included diesel oil, toluene, dichloromethane, petroleum ether, n-hexane and chloroform. The deposited copper mesh was used as a membrane, and a mixture of the oil colored with oil red (except for the diesel oil) and water was poured slowly into a beaker through the coated copper mesh. The separated oil and water were collected with a beaker and a Petri dish, respectively, to measure the separation efficiency. The mass of the oil before mixing and after separation was weighed to evaluate the separation efficiency, which was calculated as the mass ratio of the oil collected from the beaker to the original oil before mixing.Characterization
The morphologies of the samples were observed using a field-emission scanning microscope (SEM equipped with EDX, Hitachi S-4800, Japan) and an atomic force microscope (AFM, SPI3800N, Japan). The chemical composition was characterized by Fourier transform infrared (FTIR, Bruker Vector 22, Germany), energy-dispersive X-ray (EDX) and X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, America, Al Kα X-ray source, hν = 1486.6 eV). A Data Physics System OCA20 from Germany was used to measure the water contact angle (WCA) and sliding angle (SA) at room temperature. The volumes of the drops used to measure the WCA were 4 µL, and were 10 µL for the measurement of the SA. Five different positions were measured for all the samples.Results and discussion
Silicone emits white smoke when it burns. We held a glass slide exposed to the white smoke, and small white particles (WPs) were deposited on the glass surface and formed a layer. In particular, we found that the white particles layer (WPL) was water-repellent. When a water droplet was dripped on the WPL, it bounced on the surface and readily fell off.Silica nanoparticles and the WPs were characterized by FTIR spectra as shown in Fig. 1a. The absorption band at 1104 cm−1 is attributable to the asymmetric stretching vibration of Si–O–Si, the one at 807 cm−1 is assigned to the symmetric vibration of Si–O–Si and the peak at 471 cm−1 corresponds to the bending vibration of Si–O–Si.29,30 These are characteristic peaks of silica, which appear the same in the spectrum of the WPs. An EDX spectrum of the WPs is presented in Fig. 1b, indicating that the elements were silicon and oxygen and that the atomic ratio of silicon to oxygen was nearly 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. The atomic ratio was further confirmed by XPS, which showed that the silicon atomic percentage was 30.93% and the oxygen atomic percentage was 69.07%. The results demonstrate that the WPs collected from the silicone combustion smoke were silica.
7. The atomic ratio was further confirmed by XPS, which showed that the silicon atomic percentage was 30.93% and the oxygen atomic percentage was 69.07%. The results demonstrate that the WPs collected from the silicone combustion smoke were silica.
The water repellence of the WPL and its facile preparation by smoke deposition aroused our interest. Since the silicone combustion product can be utilized to fabricate superhydrophobic coatings,20 as we previously reported, it is satisfactory to make use of the emitted smoke, which means that the discarded silicone can be recycled for full utilization. However, there is a limitation in the direct utilization of WPs to prepare superhydrophobic surfaces, in that the binding force between the WPs and the substrate is so fragile that the WPs can easily be peeled off by water drops. Thus, additional treatments are required to create a more robust coating surface. We have used a silicone coating previously31 as a binder to fix carbon nanoparticles produced by flame soot to prepare robust superhydrophobic surfaces. Paraffin wax32 was also reported to fix candle soot to fabricate a relatively robust superhydrophobic surface. However, the melting point of paraffin wax is low, which hinders the practical application of the superhydrophobic surface. Silicone elastomers’ excellent heat resistance and long-term stability enable them to be widely used.31 Thus, a silicone coating was still chosen to fix the WPs to prepare superhydrophobic surfaces in this work.
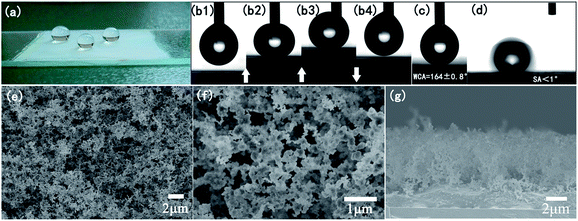
The water repellency of the coating is highlighted in Fig. 2a: water droplets exhibited spherical shapes on the WPs/silicone/glass (smoke-deposited coating) surface. After removing the water droplets, no water traces were left on the surface. Fig. 2b1–b4 display a contact–deformation–departure process of a 4 µL water droplet suspended on a syringe with the smoke-deposited coating surface. The water droplet contacted with the coating so heavily that it deformed. However, after moving the smoke-deposited coating down, the water droplet could detach from the coating surface and remain suspended on the syringe. The coatings exhibited very small sliding angles of less than 1°, as shown in Fig. 2d. The extraordinary water repellency made it hard to measure the WCAs without water droplets becoming suspended on the syringe. Before smoke deposition, the static WCA of the pure silicone coating was about 118°. As shown in Fig. 2c, the WCA of the smoke-deposited coating reached 164 ± 0.8°, demonstrating excellent superhydrophobicity. Fig. 2e and f present SEM images, at different magnifications, of the smoke-deposited coating surface. Nanoparticles with various diameters strung into lines like intertwining pearl necklaces or grew into micro-scale clusters with a high porosity, forming an interconnected network. The irregular agglomerates, consisting of the nanoparticles adhered to silicone coatings, endowed a surface hierarchical morphology with dual-scale roughness. Owing to an increase in surface roughness by the formation of granular aggregates of hydrophobic smoke particles, a superhydrophobic surface could be created. It can be seen from Fig. 2g that the thickness of the WPL was about 6 µm. It was hard to ensure that the thickness was kept at a fixed value, since the process was manual. The thickness of the WPL ranged from 6–10 µm.
Fig. 3a presents the thermal stability of the smoke-deposited coating. At a temperature of 200 °C, the heating time was varied from 10 to 180 min, resulting in a negligible influence on the WCAs. The WCAs still remained above 164° with increasing heating time to 180 min, which means that the smoke-deposited coating exhibited the property of heat resistance. That is because silicone is a cross-linked polymer possessing a high maximum application temperature of more than 250 °C.
A water-flow impact test was carried out to study the stability of the smoke-deposited coating. The water flow from a faucet with a diameter of about 3 mm impacted the samples for different times at the position of about 10 cm height. As shown in Fig. 3b, the WCAs changed very slightly from an initial value of 164.8° to 162.7° after 10 min water impact. The WPs which adhered and were fixed well on the silicone coating were strong enough to resist water impact for a certain time, but the WPs connected by bonding forces between the particles were not tough enough to resist water impact for a long time. Thus, the WPL tended to get thinner with increasing water impact time, as shown in Fig. 4a3–d3. When water flow struck the coating surface for a short time (1–3 min), some particles with the weakest binding forces were stripped away, resulting in a coarser morphology with obvious micro-scale structure compared to pristine densely-packed WPs, as shown in Fig. 4a1 and a2. The generation of more obvious micro- and nano-scale rough structure led to the tiny increase in the WCAs of the coating surface after 1–3 min water impact. With increasing water impact time, the WPs were peeled away increasingly, as presented in Fig. 4a–d. The particle agglomerates decreased from totally covering the silicone coating to being evenly distributed on the silicone, accompanied by homogeneous uncovered silicone grooves. After 10 min water impact, it can be clearly seen that the micro-scale agglomerates had almost vanished and only the nanoparticles fixed on the silicone were left. Without the dual-scale hierarchical structure combining nanostructure with micro-scale roughness, the WCAs decreased slightly. However, the nanoparticles fixed well on the silicone could be maintained to provide a rough surface structure, supporting the coatings with superhydrophobicity. Thus, the WCA of the coating surface after 10 min water impact still remained at around 162°. The roughness can be further confirmed by the AFM images. Fig. 5 shows an AFM image and a three-dimensional AFM image of the superhydrophobic coating surface after being impacted by water for 10 min. The scan area was 3 × 3 µm2. The RMS of the surface was measured to be over 300 nm, indicating that the surface had a considerable roughness. However, the coatings which had been subjected to shorter water impact times were much harder to measure. This may have been due to the larger roughness, which can be seen from the SEM images (Fig. 4a3–d3).
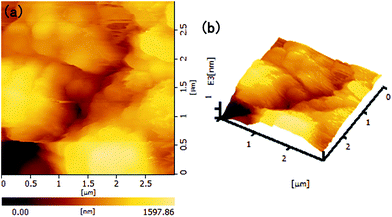 | ||
| Fig. 5 (a) AFM image and (b) three-dimensional AFM image of the smoke-deposited coating surface after water impact for 10 min. | ||
As shown in Fig. 6a, upon being totally soaked in water, the smoke-deposited coating surface acted like a silver mirror when viewed at a glancing angle. The mirror-like surface could reflect the shape of a nearby pen, indicating that air was trapped by the porous rough morphology and that solid–liquid–air interfaces were established.33 Notably, after 24 hours immersion, the silver mirror-like phenomenon on the coating surface still remained and reflected the appearance of fingers in front of it, confirming that the air-trapped rough structure still existed (Fig. 6b). It can be clearly seen by SEM observation that the nanoparticle agglomerates were still evenly distributed on the silicone surface after 24 hours of water immersion (Fig. 6c). In practical use, coatings may suffer from similar water soaking, resulting in the degradation of superhydrophobicity and hindering practicability. Some investigations of superhydrophobic coatings study their water-immersion stability by drying the coatings after immersion, but herein, we directly tested the WCA variation after water immersion without a drying process. Fig. 3c shows the change in the WCA with regard to immersion time in tap water for the smoke-deposited coating. After 249 hours, the superhydrophobicity still remained, with a high WCA value. Excluding the test error, the WCA kept nearly constant during the initial 249 hours.
At a large magnification, as shown in Fig. 6d and in the cross-section in Fig. 6e, it can be seen that although the overall nanoparticle agglomerates became flat at the top position (having been compressed by water), a porous coarse surface was retained. This can be attributed to the water pressure and weak van der Waals interactions,34 which did not change the constituents and surface morphology significantly, resulting in retention of water repellence with a constant WCA. With increasing immersion time, the WPL became thinner due to the water pressure, causing degradation of superhydrophobicity at some positions. Over a test for 380 hours, although the coating surface was dominated by superhydrophobicity, some point positions lost superhydrophobicity, such that the WCA dropped below 150° as shown in Fig. 3c. The area that lost superhydrophobicity expanded from points to the surface, and the SA became so high that water droplets stayed on the surface even upon inversion, indicative of loss of water repellence. The test reveals that the smoke-deposited coating possessed good stability to resist water soaking for a certain time.
The smoke-deposited coating with its excellent water repellence, heat durability, water impact and immersion stability demonstrates the feasibility of preparation of superhydrophobic surfaces, which can be used in the field of water-repellent coatings. Besides this, we found that the coating could not resist the attack of oil and various organic solvents, which was confirmed by oil instantly spreading on the coating surface when oil was dripped on it. In other words, the coating possesses superhydrophobicity and superoleophilicity. Inspired by oil spill incidents and by the extensive research on oil–water separation materials, we applied the smoke deposition method to prepare superhydrophobic and superoleophilic meshes based on copper meshes. When copper mesh with a small pore size (150 µm and below) was coated with liquid silicone, it was not easy to keep the pores unblocked due to the capillary force. However, when the pore size was over 250 µm, the superhydrophobicity and separation efficiency of the mesh declined because the pores were too large to confine the air into the structure. So, here, we chose copper mesh with a pore size of 180 µm as the substrate.35
SEM images of the copper meshes before and after smoke deposition are presented in Fig. 7a1–a3 and b1–b3. The initial copper mesh wires were quite smooth with slight metal scratches. After coating with silicone and smoke deposition, nanoparticles were aggregated and homogeneously distributed on the mesh surface, reducing the hole size of the mesh from 180 µm initially down to about 120–170 µm. The difference in the reduced hole size is mainly attributed to variation in the thickness of the silicone coating, not the WPL, the thickness of which was limited as aforementioned. But most importantly, the silicone coating and WPs did not totally block the mesh holes, guaranteeing the penetrability of wettable liquid. Obviously, the same porous coarse structure as that of the surface morphology of the smoke-deposited coating was formed on the mesh, endowing the mesh with improved wettability that can be applied to oil–water separation.
The oil–water separation experiment was performed as shown in Fig. 7c–f. The coated mesh, the edges of which were folded to make a box-like filter and a gap left in a corner to allow water to flow away, was put on the top of a beaker. When a mixture of diesel oil and water (Fig. 7c) was poured on the mesh, the oil immediately permeated through the mesh and streamed into the beaker, while the water was excluded above the mesh and converged to flow away from the gap, as shown by the red arrow in Fig. 7d. Fig. 7e clearly displays the excluded water on the mesh, and, notably, an oil droplet suspended under the mesh as highlighted by the red circle, demonstrating the reliability and convenience of separating oil–water mixtures. The diesel oil was efficiently collected in the beaker (Fig. 7f). We further investigated the separation efficiency of the smoke-deposited mesh by collecting and weighing the oil collected in the beaker. As shown in Fig. 7g, different types of oils and organic solvents, i.e. diesel oil, toluene, dichloromethane, petroleum ether, n-hexane and chloroform, could be separated by the smoke-deposited mesh, at an efficiency of more than 93%. In addition, the as-prepared smoke-deposited mesh exhibited a high separation efficiency after 10 cycles for different oils (Fig. 7h). The results confirm that the superhydrophobic and superoleophilic smoke-deposited copper mesh possessed good recyclability for oil–water separation. More importantly, the degraded superhydrophobic mesh could regain excellent water repellence after recoating with silicone and smoke deposition. The fabrication process can also be applied to general metal meshes, such as stainless steel mesh. Employing the strategy of designing oil collection devices reported in the literature,21,26 box-like oil collector and self-driven oil collector devices could be created utilizing the smoke-deposited mesh, realizing the direct application in dealing with large-scale offshore oil spills. This simple and versatile fabrication approach of oil–water separation meshes provides a potential avenue to the large-scale preparation of superhydrophobic meshes for oil spill applications.
Conclusions
A superhydrophobic smoke-deposited coating has been rapidly fabricated by the use of silicone combustion smoke and silicone as a binder. FTIR, EDX and XPS analyses confirmed that the silicone combustion smoke is composed of silica nanoparticles. SEM images showed that nanoparticle agglomerates formed and fixed on the silicone to construct a dual-scale rough morphology, endowing the surface with superhydrophobicity and with excellent stabilities towards heat, water impact and water immersion. This method avoids the drawbacks of conventional oil-separation materials in the fabrication process, such as high cost, complex procedures, being time-consuming, employing specific equipment and requiring materials including toxic organic solvents, and offers a simple and recyclable preparation approach. By this simple method, a selective wettability (superhydrophobic and superoleophilic) copper mesh has been fabricated. The smoke-deposited mesh achieves high separation efficiencies of over 93% for various oils, and shows excellent reusability, maintaining high separation efficiency over 10 cycles. The used mesh can be refreshed with excellent water repellence by recoating with silicone and smoke deposition. This fabrication method of oil–water separation meshes has scale-up prospects in practical applications for efficient oil spill removal and oil purification.Acknowledgements
We gratefully acknowledge that the project was supported by the Natural Science Foundation of Zhejiang Province (No. LY13E030001).Notes and references
- P. Lenz, Adv. Mater., 1999, 11, 1531 CrossRef CAS
.
- D. Yoo, S. S. Shiratori and M. F. Rubner, Macromolecules, 1998, 31, 4309 CrossRef CAS
.
- J. Lahann, S. Mitragotri, T. Tran, H. Kaido, J. Sundaram, I. S. Choi, S. Hoffer, F. A. Somorjai and R. Langer, Science, 2003, 299, 371 CrossRef CAS PubMed
.
- C. Dorrer and J. Rühe, Soft Matter, 2009, 5, 51 RSC
.
- L. Shen, H. L. Ding, Q. H. Cao, W. C. Jia, W. Wang and Q. P. Guo, Carbon, 2012, 50, 4284 CrossRef CAS
.
- Y. M. Zheng, H. Bai, Z. B. Huang, X. L. Tian, F. Q. Nie, Y. Zhao and L. Jiang, Nature, 2010, 463, 640 CrossRef CAS PubMed
.
- S. Srinivasan, V. K. Praveen, R. Philip and A. Ajayaghosh, Angew. Chem., Int. Ed., 2008, 47, 5750 CrossRef CAS PubMed
.
- Y. B. Park, H. Im, M. Im and Y. K. Choi, J. Mater. Chem., 2011, 21, 633 RSC
.
- G. Y. Qing, X. Wang, H. Fuchs and T. L. Sun, J. Am. Chem. Soc., 2009, 131, 8370 CrossRef CAS PubMed
.
- B. Wang, W. X. Liang, Z. G. Guo and W. M. Liu, Chem. Soc. Rev., 2015, 44, 336 RSC
.
- E. Celia, T. Darmanin, E. T. Givenchy, S. Amigoni and F. Guittard, J. Colloid Interface Sci., 2013, 402, 1 CrossRef CAS PubMed
.
- A. Oláh, H. Hillborg and G. J. Vancso, Appl. Surf. Sci., 2005, 239, 410 CrossRef
.
- H. Hillborg and U. W. Gedde, IEEE Trans. Dielectr. Electr. Insul., 1999, 6, 703 CrossRef CAS
.
- B. Cortese, S. D’Amone, M. Manca, I. Viola, R. Cingolani and G. Gigli, Langmuir, 2008, 24, 2712 CrossRef CAS PubMed
.
- C. R. Crick, J. C. Bear, P. Southern and I. P. Parkin, J. Mater. Chem. A, 2013, 1, 4336 CAS
.
- J. L. Yong, F. Chen, Q. Yang, D. S. Zhang, H. Bian, G. Q. Du, J. H. Si, X. W. Meng and X. Hou, Langmuir, 2013, 29, 3274 CrossRef CAS PubMed
.
- M. Ouyang, R. J. Muisener, A. Boulares and J. T. Koberstein, J. Membr. Sci., 2000, 177, 177 CrossRef CAS
.
- J. Frommer, D. Sampson, R. Campbell, D. Miller, C. Hawker, V. Lee and R. D. Miller, Chem. Mater., 1998, 10, 3895 CrossRef
.
- M. D. Nyman, S. B. Desu and C. H. Peng, Chem. Mater., 1993, 5, 1636 CrossRef CAS
.
- L. Shen, W. L. Qiu, B. Liu and Q. P. Guo, RSC Adv., 2014, 4, 56259 RSC
.
- J. L. Song, S. Huang, Y. Lu, X. W. Bu, J. E. Mates, A. Ghosh, R. J. Ganguly, C. Carmalt, I. P. Parkin and W. J. Xu, ACS Appl. Mater. Interfaces, 2014, 6, 19858 CAS
.
- C. F. Wang and S. J. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 8861 CAS
.
- P. Calcagnile, D. Fragouli, I. S. Bayer, G. C. Anyfantis, L. Martiradonna, P. D. Cozzoli, R. Cingloani and A. Athanassiou, ACS Nano, 2012, 6, 5413 CrossRef CAS PubMed
.
- A. V. Rao, N. D. Hegde and H. Hirashima, J. Colloid Interface Sci., 2007, 305, 124 CrossRef PubMed
.
- L. Feng, Z. Y. Zhang, Z. H. Mai, Y. M. Ma, B. Q. Liu, L. Jiang and D. B. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012 CrossRef CAS PubMed
.
- F. J. Wang, S. Lei, M. S. Xue, J. F. Ou, C. Q. Li and W. Li, J. Phys. Chem. C, 2014, 118, 6344 CAS
.
- C. X. Wang, T. J. Yao, J. Wu, C. Ma, Z. X. Fan, Z. Y. Wang, Y. R. Cheng, Q. Lin and B. Yang, ACS Appl. Mater. Interfaces, 2009, 1, 2613 CAS
.
- X. Y. Zhou, Z. Z. Zhang, X. H. Xu, F. Guo, X. T. Zhu, X. H. Men and B. Ge, ACS Appl. Mater. Interfaces, 2013, 5, 7208 CAS
.
- K. Y. Jung and S. B. Park, Appl. Catal., B, 2000, 25, 249 CrossRef CAS
.
- C. Anastasescu, M. Anastasescu, V. S. Teodorescu, M. Gartner and M. Zaharescu, J. Non-Cryst. Solids, 2010, 356, 2634 CrossRef CAS
.
- L. Shen, W. Wang, H. L. Ding and Q. P. Guo, Appl. Surf. Sci., 2013, 284, 651 CrossRef CAS
.
- K. Seo, M. Kim and D. H. Kim, Carbon, 2014, 68, 583 CrossRef CAS
.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 0546 RSC
.
- L. Boinovich, A. M. Emelyanenko and A. S. Pashinin, ACS Appl. Mater. Interfaces, 2010, 2, 1754 CAS
.
- D. L. Tian, X. F. Zhang, X. Wang, J. Zhai and L. Jiang, Phys. Chem. Chem. Phys., 2011, 13, 14606 RSC
.
| This journal is © The Royal Society of Chemistry 2015 |